Heterogeneity and multiscale complexity of tissues
Biological tissues are complex assemblies of diverse cell types, extracellular matrix, and molecular components that contribute to the specific functions of each tissue type. Due to their heterogeneity and multiscale complexity, alterations in tissue mechanical properties occur from subcellular to tissue levels and are influenced by age, disease development, and physiological conditions. Conventional techniques struggle to assess these properties over large areas with high resolution.
This challenge is addressed by measuring mechanical properties across extensive tissue areas while providing high-resolution details of tissue mechanical heterogeneities at a fine scale. This is especially essential when dealing with heterogeneous tissue regions, which may possess distinct mechanical properties.
Stiffness is an indicator of the disease’s pathophysiology. Measuring tissue stiffness helps evaluate early detection, monitor disease progression, and explore potential therapies.
Loss of tissue viscoelasticity impairs organ functionality. Understanding tissues’ viscoelastic behavior can provide insights into disease mechanisms and support treatments to restore tissue mechanics.
Topographical cues indicate structural changes in tissues that can be linked to pathological conditions.
Stress relaxation characterizes how tissues reduce stress under constant strain over time. Measuring stress relaxation provides insights into the time-dependent mechanical properties of tissues and how they respond to mechanical loads.
Creep describes the gradual deformation of tissues under a constant load. Evaluating creep behavior in tissues helps us understand the long-term mechanical changes associated with disease progression and treatment effects.
Incorporating mechanical measurements will:
• No need for extensive sample preparation.
• Preserve tissue integrity through non-destructive analysis at various workflow stages.
• Correlate mechanical data with subsequent downstream experiments.
• No prior knowledge or selection of specific proteins or genes.
• Evaluate the overall impact of mechanical changes when multiple protein expressions are modified.
• Support the study of disease mechanisms by comparing healthy and pathological conditions.
• Replicate natural tissues in engineered models.
Advance early-phase testing and validation of potential therapies.
• Provide a broader indicator of disease status than individual proteins, which offer a limited perspective on disease development.
• Facilitate early disease detection.
• Measure mechanics from sub-cellular to tissue levels.
• Identify localized tissue mechanical heterogeneities at the cell scale.
We enable researchers to get the most out of their time and efforts by providing solutions that meet their diverse and versatile needs.
Powerful insights in a small package. Discover our compact, standalone Piuma platform.

Combine unique mechanical insights with the imaging equipment of your choice. Our Chiaro platform is the perfect collaborator.

Discover high-throughput mechanical screening that seamlessly integrates with existing biological workflows, effortless correlation, offering high resolution and reproducibility.

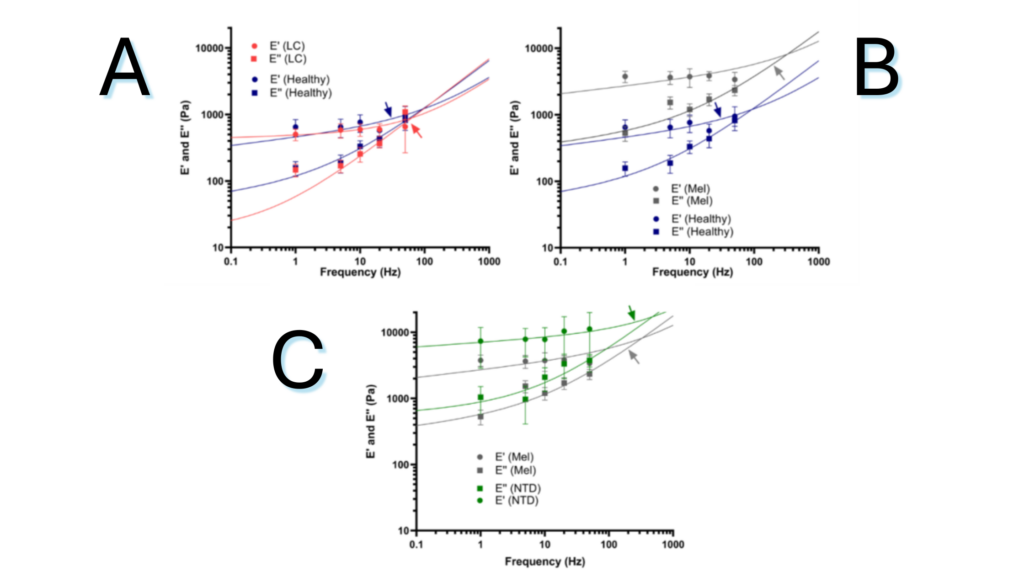
Figure 1.1. Frequency dependent loss (E’) and storage (E”) modulus as measured by dynamic mechanical analysis (DMA) of the ECM of healthy lung and the ECM-rich regions of (A) lung carcinoma (CAR) and (B) melanoma (MEL) lung macrometastasis. (C) DMA of the ECM of ECM-rich regions of MEL lung macrometastasis in NTD treated and non-treated mice.
Note. Adapted from “Lung Micrometastases Display ECM Depletion and Softening While Macrometastases Are 30-Fold Stiffer and Enriched in Fibronectin”, Narciso, M., 2023, Cancers (Basel). 2023 Apr 21;15(8):2404. doi: 10.3390/cancers15082404. © The Author(s) 2023. This is an open access article under the CC BY license.
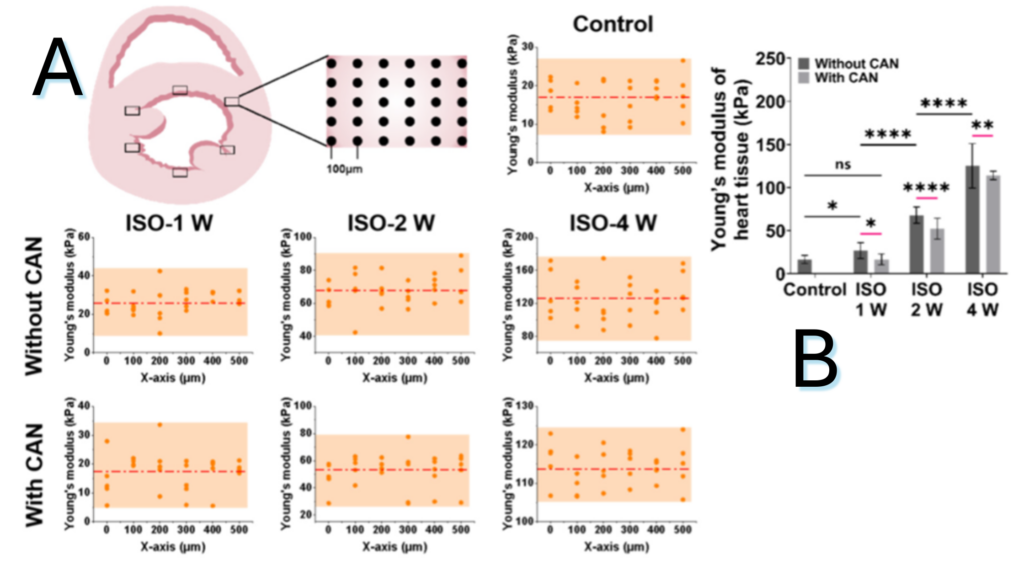
Figure 1.2. Characterization results of myocardial tissue stiffness and collagen deposition in myocardial fibrosis rats without and with CAN treatment. (A) Stiffness diagrams of tissue samples were obtained based on their Young’s moduli measured by nanoindentation instrument. (B) Statistical histograms of tissue stiffness of fibrotic myocardium.
Note. Adapted from “Effect of Extracellular Matrix Stiffness on Candesartan Efficacy in Anti-Fibrosis and Antioxidation”, Zhu, T, 2023, Oxidative Stress in Cardiac Disease. 2023; 12(3), 679. doi: 10.3390/antiox12030679. © 2023 by the authors. Licensee MDPI, Basel, Switzerland. This is an open access article under the CC BY license.
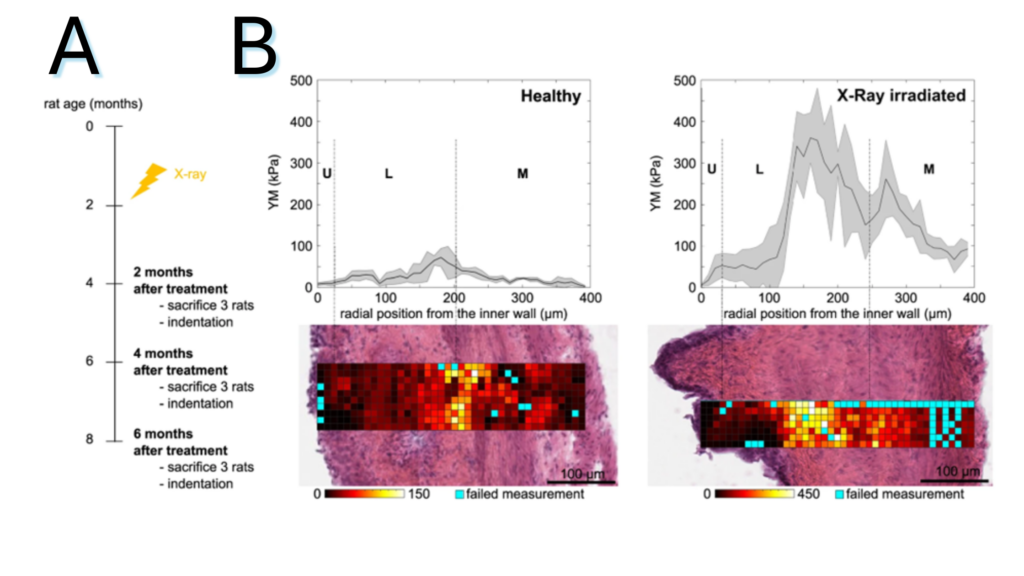
Figure 2.3. (A) Schematic representation of the experiment: X-ray radiotherapy is used to induce actinic cystitis on the bladder (B) Representative bladder wall stiffness gradient collected 4 months after treatment: X-rays cause a stiffening of the whole bladder wall compared to non-treated healthy animals. Mechanical spatial differences within the fibrotic bladder are maintained and associated to the different tissue layers (U: urothelium, L: lamina propria, M: muscle).
Note. Adapted from “Micro-mechanical fingerprints of the rat bladder change in actinic cystitis and tumor presence”, Martinez-Vidal, L., 2023, Commun Biol., 6(1):217. doi: 10.1038/s42003-023-04572-0.19. © The Author(s) 2023. This is an open access article under the CC BY license.





Whether your focus lies on mechanical measurements and characterization at the cell scale, or you work with muscle tissues, our platforms offer you precise, fast, and accurate outcomes. Discover more about how our products can help you accelerate and achieve your research goals.
With a wide range of application areas, across an array of samples, for various disease areas and testing needs, we can provide you with precise insights into mechanical cues to advance your translational research. Learn more about our applications here.
We are a growing team of 60+ passionate people, headquartered in Amsterdam, the Netherlands. Learn more about our journey so far, meet our team of professionals, and our career opportunities.
Resources
Contact