Welcome to our latest dive into cutting-edge research! Today, we’re diving into a fascinating study published at the Translational Research exploring changes in cardiac-specific decellularized matrices under fibrotic conditions. Don’t let the scientific jargon scare you off—this research conducted by Giancarlo Forte‘s group has some exciting implications for understanding and treating heart disease.
Decellularized matrices: a quick overview
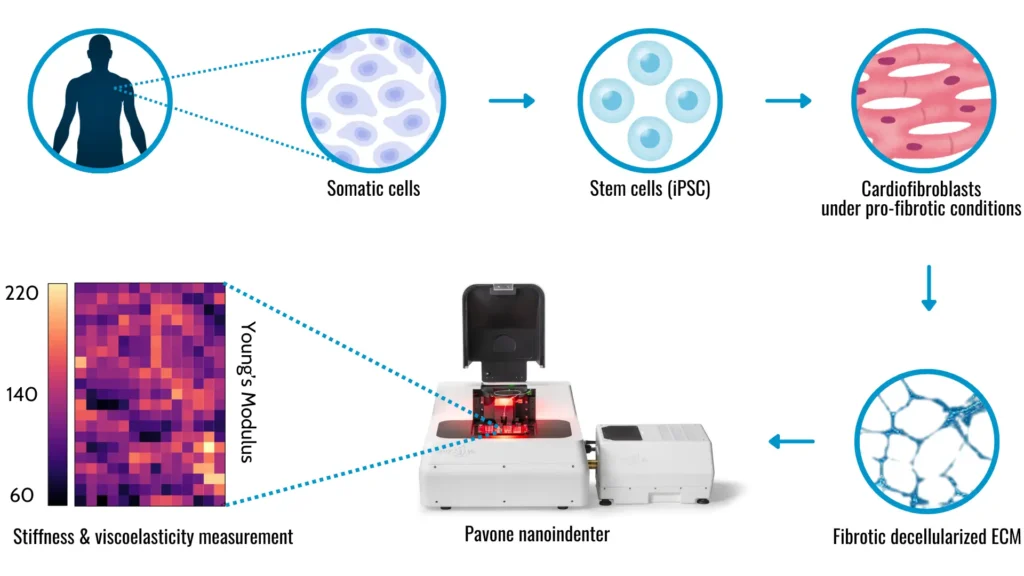
First, let’s break down what we’re talking about. Decellularized matrices are scaffolds made from cells with all their cellular components removed. Think of them as the structural “skeletons” cells use to build tissues. In this study, researchers focused on these matrices derived from induced pluripotent stem cells (iPSCs), which are a type of stem cell that can turn into almost any cell type in the body. These cells can be differentiated in cardiac fibroblasts and secrete fibrotic matrices under pro-fibrotic experimental conditions. After decellularization, these matrices serve as in vitro substrates for cardiomyocyte cultures (heart cells), facilitating the study of critical biological alterations induced by fibrosis.
What is fibrosis?
Fibrosis occurs when excess fibrous connective tissue builds up in an organ. This accumulation can cause serious trouble in the heart, contributing to diseases like heart failure. Therefore, understanding how fibrosis changes these scaffolds can provide new insights into cardiac health.
Pavone: high-tech mechanical screening platform
To investigate these changes, the researchers used the high-throughput technology Pavone. This tool allowed them to study the mechanical properties of the extracellular matrix (ECM)—the non-cellular component of tissues that provides structural and biochemical support to cells. Pavone helped them link these mechanical properties to the structural components of the matrices, creating a detailed map of how fibrosis affects the ECM.
Fibrosis changes the mechanics of ECM
The study revealed some crucial insights. Under pro-fibrotic conditions, the ECM’s elastic and viscoelastic properties changed significantly. These changes were associated with significant alterations in the ECM structure and the composition of proteins. This is crucial because the mechanical properties of the ECM are vital for tissue function, especially in the heart.
One of the standout points from this research is that examining the mechanical properties of the ECM offers a more comprehensive view of fibrosis. Think of it like trying to understand a car’s performance by looking at the whole engine rather than just one part. By assessing the overall mechanical condition, researchers can gain better insights into the progression of fibrosis and its impact on heart health.
A potential for new therapies
These findings open new avenues for developing better in vitro laboratory models that more accurately mimic the human physiology and highlight potential new targets for drug development to reduce cardiac fibrosis and its complications. By emphasizing the significance of ECM remodeling in cardiac dysfunction, this study provides a deeper understanding of the role of fibrosis in heart disease. This comprehensive approach offers a broader perspective than traditional methods, potentially leading to significant advancements in cardiac health research and more effective treatments for heart diseases.
Disclaimer for blog use: This blog simplifies complex scientific information for general understanding. The content is meant for educational purposes, highlighting advances in mechanobiology and disease investigation. Readers are encouraged to consult scientific articles for detailed insights and to recognize the broader context of this research within the scientific community: https://www.translationalres.com/article/S1931-5244(24)00144-0/fulltext



