by Optics11 Life
© 2024 Optics11 Life B.V.
This application note is relevant for researchers and scientists in tissue engineering, regenerative medicine, and organoid cell culture aiming to optimize experimental conditions and improve the consistency and reliability of biological assays in tissue engineering applications.
Matrigel®, widely used in organoid cell culture, is a hydrogel rich in extracellular matrix proteins to simulate in vivo cell environments. However, Matrigel® is subject to batch-to-batch variability in protein concentration and composition, potentially impacting its mechanical features such as stiffness and viscoelasticity and subsequently influencing its interaction with biological systems. Additionally, studies have reported diverse mechanical properties for Matrigel®, suggesting the need for standardized handling protocols and measurements. In this context, ensuring reproducibility and functionality of biological experiments demands precise mechanical measurements to optimize experimental conditions. Using the Pavone mechanical screening platform, this application note evaluates Matrigel’s mechanical properties across different batches over time.
Introduction
Matrigel® is a hydrogel widely employed as an in vitro culture substrate in various applications, such as tissue engineering and regenerative medicine1. It is derived from a basement membrane matrix secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells, rich in extracellular matrix (ECM) proteins including laminin, collagen IV, heparan sulfate proteoglycans, entactin/nidogen, and several growth factors1,2. Matrigel® guides cell function and tissue development by mimicking the native ECM and providing mechanical stimuli to 3D cell constructs3.
However, different batches of Matrigel® exhibit variations in biochemical composition and protein concentration, leaving uncertainty about the uniformity of mechanical properties across experiments4,5. This variability can influence the stiffness and viscoelasticity of Matrigel®, which affect cell–matrix interactions6. Thus, exploring Matrigel® mechanics is essential for replicating the complex microarchitecture and physiological functions of tissues and designing in vitro models that closely resemble in vivo conditions.
Several studies have investigated the mechanical properties of Matrigel® using distinct methodologies. For instance, rheometric analysis of Corning® Matrigel® indicates a Young’s modulus range of approximately 300–600 Pa, dependent on gel concentration1. However, extrapolating this data to in situ cell culture conditions poses challenges due to inherent disparities between rheometric measurements and cellular environments. Alternatively, optical tweezers revealed that the viscoelastic properties of Matrigel® change as a function of time and polymer concentration. Observations identified a transition from a viscous to an elastic state after a few hours, with higher polymer concentrations correlating to increased matrix elasticity7.
However, reports on the mechanical properties of Matrigel® remain discrepant. Under comparable conditions, two Atomic Force Microscopy (AFM) studies reported divergent Young’s modulus values of approximately 400 Pa4 and 33 Pa8. Interestingly, a Piuma nanoindenter study reported a Young’s modulus of approximately 600 Pa9. Since actual protein concentrations are undisclosed in most in situ studies, it is challenging to compare experimental results. Therefore, ensuring the reproducibility and functionality of biologically relevant Matrigel® requires accurate and sensitive mechanical measurements to control their mechanics and optimize experimental conditions.
In this application note, we assessed the mechanical properties of the Matrigel® using Pavone, a mechanical screening platform for non-destructively characterizing soft biomaterials. First, we investigated batch-to-batch variability in mechanical properties and its dependence on protein concentration. Second, we assessed the stability of Matrigel’s mechanical properties by monitoring their variation over time.
Methods
Matrigel preparation
Two batches of Matrigel® (Cat# 356234, Corning, USA) were prepared following the manufacturer’s instructions: batch 1 (Lot# 3068004, 7.6 mg/ml) and batch 2 (Lot# 3033002, 9.8 mg/ml). In brief, Matrigel® was thawed overnight at 4°C, and 80 μL were placed into a well of a 96-well plate. The plates were then transferred to a 37°C incubator for 30 min to allow for the gelation of a thick hydrogel (~2.5 mm). Afterward, Dulbecco’s Modified Eagle Medium (DMEM) was added to the wells and incubated at 37°C for specified times.
Mechanical property characterization
Matrigel’s mechanical properties were measured by Pavone using a probe with a stiffness of 0.021 N/m and a tip radius of 11.5 µm. The formed hydrogels were indented 5 µm, and the Young’s modulus was determined in batches 1 and 2. The protein concentration in batch 2 was then diluted in DMEM to 7.6 mg/ml and compared to the undiluted 1. Changes in storage and loss moduli (E’ and E’’ respectively) and loss or damping factor tan (δ) (the ratio of E’ — E’’) were measured as a function of frequency (between 1 Hz and 20 Hz) using dynamic mechanical analysis (DMA). The measurements were conducted under physiological conditions in DMEM at 37°C. Data were fitted to a Hertz contact model at 2 μm using Optics11 Life DataViewer software.
Results
To examine batch-to-batch variability, we measured the Young’s modulus of batches 1 and 2. Our results showed that the stiffness of Matrigel® varies significantly between low and high protein concentrations, wherein the higher concentration provides greater matrix stiffness (Figure 1A). Additionally, we tested Matrigel® batches adjusted to the same protein concentration (7.6 mg/ml): undiluted batch 1 and diluted batch 2. Undiluted and diluted matrices at the same concentration presented no differences in batch-to-batch mechanical properties (Figure 1B).
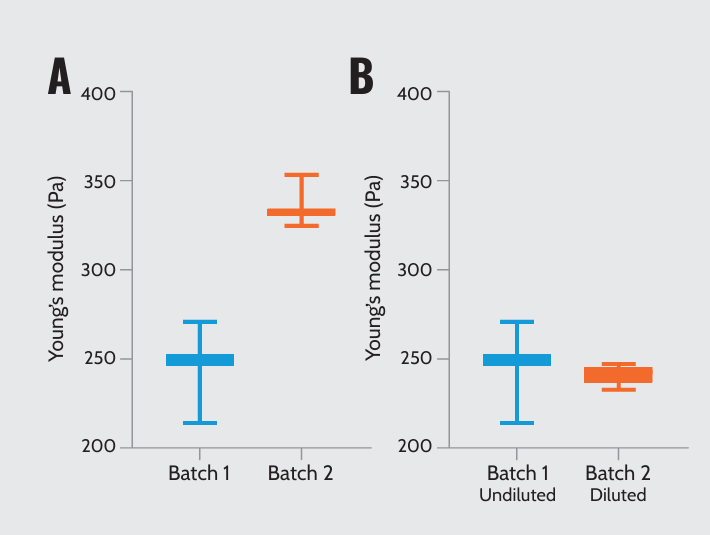
Matrigel’s mechanical properties by varying batches and protein concentrations. (A) Young’s modulus of two Matrigel® batches: batch 1 (7.6 mg/ml) and batch 2 (9.8 mg/ml). (B) Young’s modulus of two Matrigel® batches with the same protein concentration (7.6 mg/ml): undiluted batch 1 and diluted batch 2.
Notably, the surface heat map of Young’s modulus revealed a uniform distribution and low heterogeneity (spanning 100 × 100 μm) of both matrices, indicating consistent stiffness across the area (Figure 2).

Young’s modulus heat map for Matrigel® batches at the same protein concentration (7.6 mg/ml). (A) Batch 1 – undiluted. (B) Batch 2 – diluted.
To investigate whether time alters the mechanical properties of Matrigel®, we measured Young’s modulus of batches 1 and 2 over time. Our findings demonstrated that both concentrations declined in stiffness over time (Figure 3A). To elucidate this, we examined the height of the surface between time points. The observations indicated that the gel surface elevated by up to 250 μm (approximately 10%) due to swelling in the cell culture medium, possibly explaining the decline in stiffness (Figure 3B).
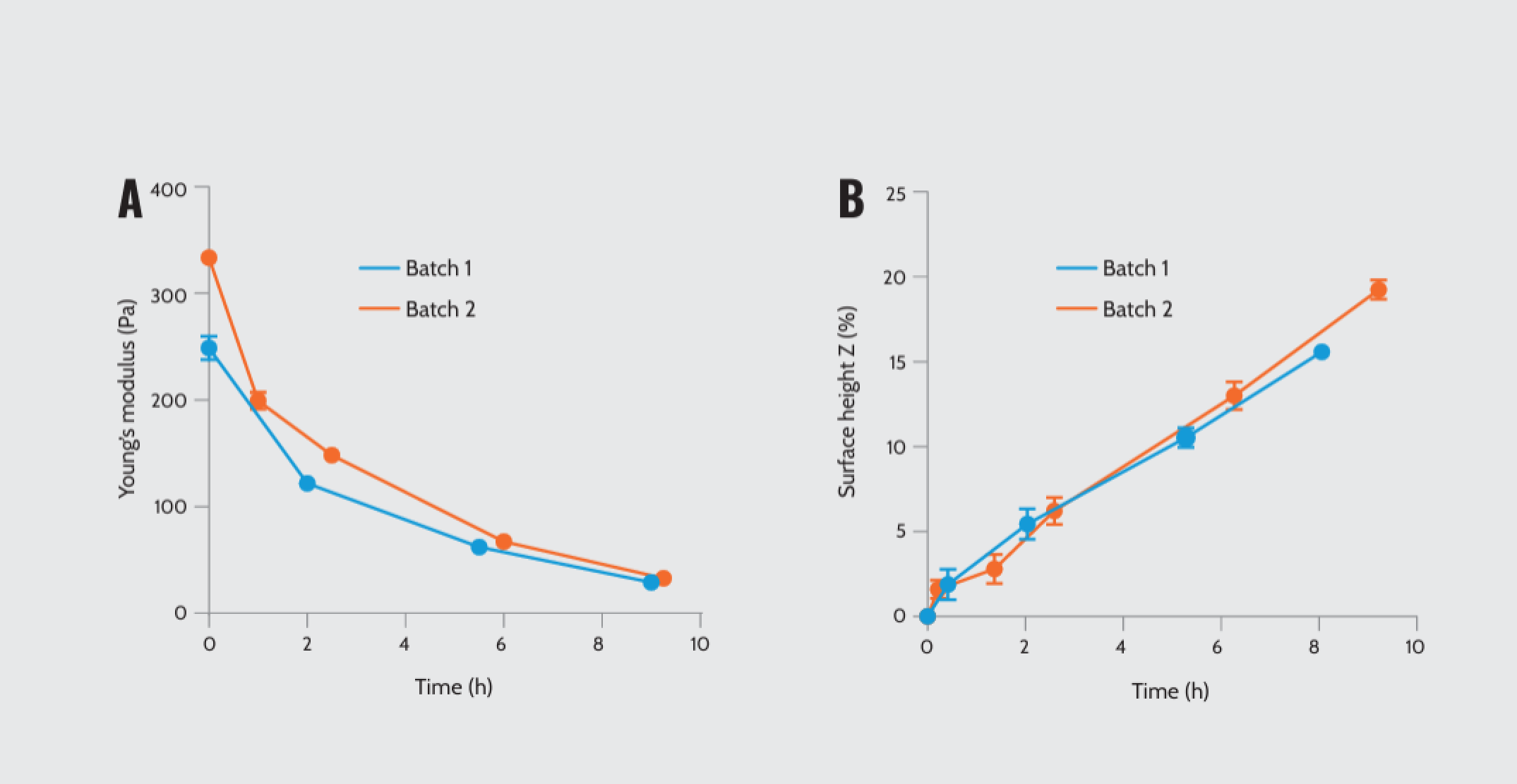
Mechanical properties of Matrigel® as a function of time. (A) Young’s modulus of two Matrigel® batches with different protein concentrations over time: batch 1 (7.6 mg/ml) and batch 2 (9.8 mg/ml). (B) Matrigel swelling analyses by plotting the height difference over time.
We also performed DMA using an oscillatory force to assess the viscoelastic properties of Matrigel®. Our results revealed a rapid decrease in storage modulus over time alongside a change in the crossover intersection, indicating a time-dependent increase in viscosity and a decrease in elasticity (Figure 4). These changes in mechanical properties can potentially influence Matrigel’s performance in biological assays or tissue engineering endeavors.
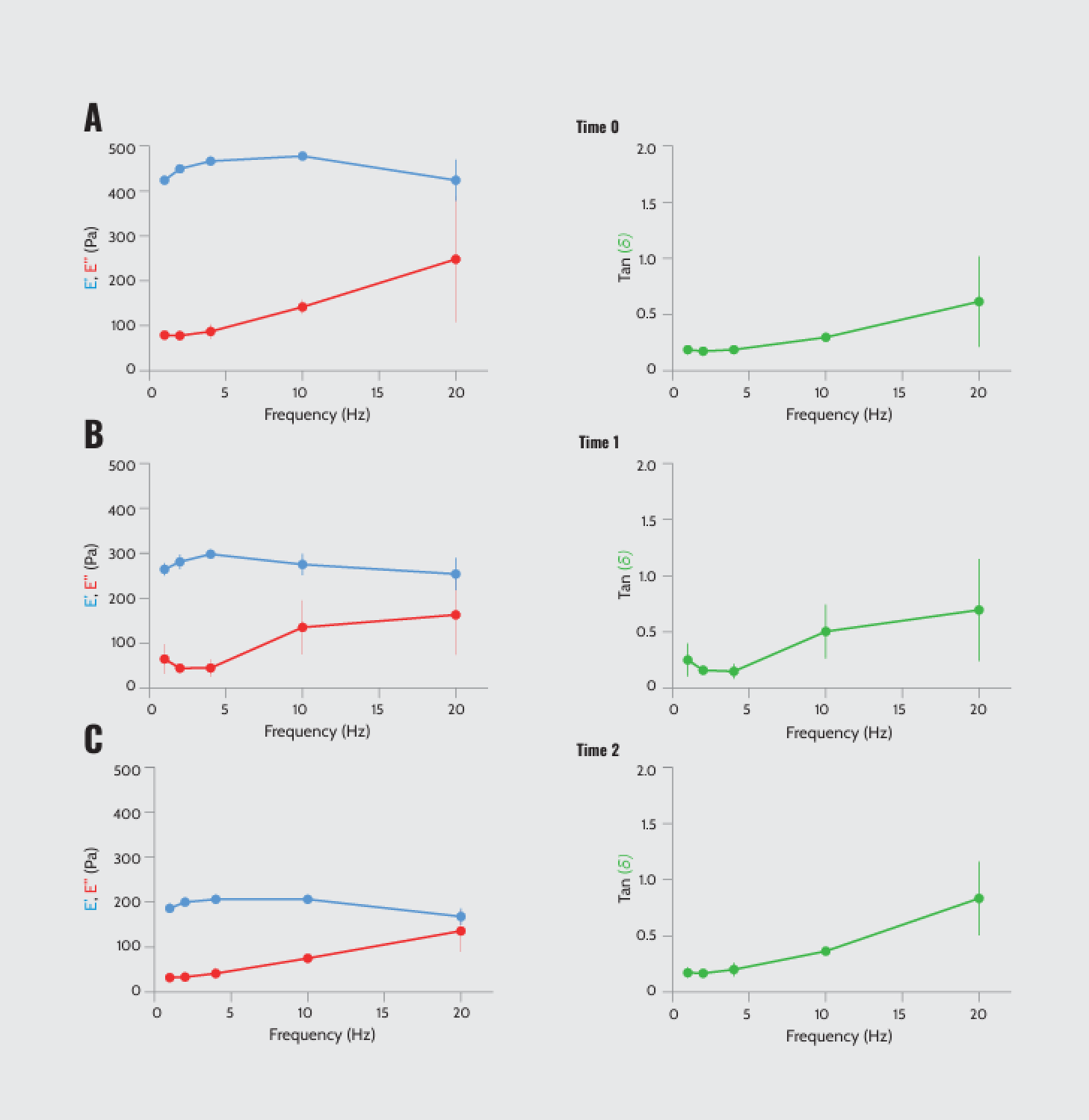
Dynamic mechanical analysis of batch 1 (9.8 mg/ml) as a function of frequency. (A–C) Storage modulus (E’), loss modulus (E’’), and loss or damping factor tan (δ) (E’/E’’) at time 0, 1 (after 1 h), and 2 (after 2.5 h). (N = 3).
Conclusion
- Batch-to-batch stiffness remains consistent when protein concentrations are equal.
- Matrigel® presents no local variation with a uniform distribution and low heterogeneity.
- The Young’s modulus of Matrigel® increases with higher protein concentrations but significantly declines over time.
- Matrigel® swells over time when submerged in a cell culture medium.
- Matrigel® demonstrates a time-dependent increase in viscosity and a decrease in elasticity.
Scientists relying on Matrigel® as a substrate for cell culture or 3D models must consider its batch- and time-dependent mechanical variability when interpreting results or designing experiments. Accordingly, Pavone measures Matrigel’s mechanical features and ensures consistency across batches, quality control, and experimental reproducibility. Different biological applications may also require Matrigel® with specific mechanical properties, making Pavone crucial for tuning the most suitable gel concentration. Therefore, integrating Matrigel® with Pavone technology will enable scientists to optimize experimental conditions, tailoring the matrix to specific requirements for the physiological environment over time.
References
1 Tuning the Elastic Moduli of Corning® Matrigel® and Collagen I 3D Matrices by Varying the Protein Concentration (CLS-AC-AN-449).
2 Heo JH, et al. Engineering the Extracellular Matrix for Organoid Culture. Int J Stem Cells 2022; 15(1):60-69. doi: 10.15283/ijsc21190.
3 Jacob S, et al. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021; 13(3):357. doi: 10.3390/pharmaceutics13030357.
4 Soofi SS, et al. The elastic modulus of Matrigel as determined by atomic force microscopy. J Struct Biol. 2009; 167(3):216-9. doi: 10.1016/j.jsb.2009.05.005.
5 Kaur S, et al. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021; 504:58-66. doi: 10.1016/j.canlet.2021.01.025.
6 Ahearne M. Introduction to cell-hydrogel mechanosensing. Interface Focus. 2014; 4(2):20130038. doi: 10.1098/rsfs.2013.0038.
7 Borries M, et al. Quantification of visco-elastic properties of a Matrigel for organoid development as a function of polymer concentration. Front. Phys. 2020; 8:579168. doi: 10.3389/fphy.2020.579168.
8 Wang Xi, et al. Modulation of designer biomimetic matrices for optimized differentiated intestinal epithelial cultures. Biomaterials 2022; 282:121380. doi: 10.1016/j.biomaterials.2022.121380.
9 Willemse J, et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 2022; 284:121473. doi: 10.1016/j.biomaterials.2022.121473.

Key takeaways:
- Unveil the secrets of Matrigel®’s batch-to-batch variability and its direct influence on the mechanical landscape of organoid cultures.
- Learn about the direct correlation between protein concentration and Matrigel®’s stiffness, which is crucial for tailoring your mechanobiology experiments.
- Discover how Matrigel® undergoes viscoelastic transformations over time, presenting challenges and opportunities in long-term organoid culture.
- Experience the precision of the Pavone high-throughput mechanical screening platform in quantifying the viscoelastic properties of Matrigel®, ensuring the reproducibility and reliability of your research.

