Quantifying the mechanical properties ofthe cell microenvironment in an organ-on-chip model via nanoindentation
by Optics11 Life | June, 2025
© 2025 Optics11 Life B.V.
Organs-on-chip combine biology and microfluidics into complex 3D systems that model tissue function in vitro. The great potential that these systems hold in replicating physiology lies in the possibility to incorporate different cell types in a dynamic, controlled microenvironment optimized in terms of biochemical and mechanical parameters.
Despite thoughtful design, factors such as fluid flow, shear stress, intrinsic material properties, and chip architecture can impact the microenvironment on-chip compared to off-chip and, in turn, affect cell behavior. This application note highlights the importance of measuring localized mechanical properties directly on-chip, using an in vitro model of the osteochondral interface.
Introduction
The functioning of any biological tissue, and the preservation of its integrity, depend on the dynamic interplay of a variety of factors. Together with different cell types, the microenvironment in which they are embedded – consisting of various signaling molecules, metabolites and, in particular, the extracellular matrix (ECM) – dictates physiological and pathological processes. The relationship between cells and their surroundings is bidirectional. While cells modify their milieu and the ECM, at the same time they respond to their composition and organization in a continuous exchange of information. This flow of communication is mediated by biochemical as well as mechanical signals. For example, physical properties of the ECM (e.g. its stiffness and viscosity, as well as its architecture) direct fundamental cell functions, such as differentiation, proliferation and migration1. Besides these passive properties, active biomechanical cues, such as tissue stretching and compression, fluid shear stress, interstitial fluid flow, and hydrostatic pressure, can also significantly influence cell behavior2,3. In turn, cells chemically and physically remodel the ECM, altering its mechanical profile. Replicating the mechanical environment, and being able to monitor its evolution, is therefore of great importance for the translatability of in vitro models2,4. However, traditional in vitro studies rely on static culture methods, wherein cells are grown on flat, rigid 2D surfaces or as scaffold-free 3D cellular aggregates. These approaches inherently lack the capacity to control or modulate biophysical and biochemical cues critical for mimicking in vivo conditions. These limitations appear even more impactful when considering biological processes to which the cell-ECM communication axis is central.
In osteoarthritis, for example, accelerated extracellular matrix remodeling and loss of integrity are at the core of pathogenesis5. Osteoarthritis is a debilitating condition affecting the joint, with increasing incidence in the growing aging population5. Accurate modelling of osteoarthritis in vitro is complicated by the fact that it originates at the osteochondral unit, the interface between the cartilage and the bone, and the interaction between these different tissues has been implicated in the disease progression5. To investigate the biology of osteoarthritis progression, accounting for the contribution of cells and ECM of both tissues in a dynamic environment, the study presented in this application note generated an organ-on-chip (OoC) model of the osteochondral interface6.
OoCs are microphysiological systems tailored to closely model the biology and function of tissues. The adoption of an OoC system offers more precise control over the cell microenvironment by granting the ability to define the culture chamber geometry, the fluid-related physical and chemical dynamics at microscale resolution and enabling the use of scaffolds with defined biochemical, structural and mechanical properties2,7,8.
While the field puts much effort into tuning the mechanical properties of the culture substrate, little is known about how deposition on-chip can influence material properties, and scientists have relied on the extrapolation from data obtained outside the assembled system. However, the translatability of these results is debatable. Hydrogels are commonly integrated into chips post fabrication, via injection of unpolymerized precursors of sufficiently low viscosity. Gelation is then induced on-chip via temperature-triggered or photo-crosslinking8. Shear forces during injection, altered temperature gradients during solidification, or the effect of illumination through different materials (air, glass, or PDMS) have the potential to affect the hydrogel’s final properties8. Additionally, once operational, the dynamic setup of the OoC exposes cells and their matrix to the physical and chemical effects of fluid flow (such as shear stress and gradients of biomolecules) that have the potential to alter the localized mechanical properties of the microenvironment (Figure 1 and ref. 3, 8). The possibility that the material properties on-chip might differ from the properties assessed off-chip complicates therefore result interpretation.
In the presented study, these limitations were addressed by assessing the localized mechanical properties of the model tissue directly on-chip using the Pavone nanoindenter. The results of this approach revealed the effects of OoC assembly on substrate mechanics, demonstrating the importance of in situ material characterization. Additionally, inflammatory conditions induced dynamic matrix remodeling, suggesting that nanoindentation can be a valuable assay for monitoring the mechanical component of disease progression in vitro.
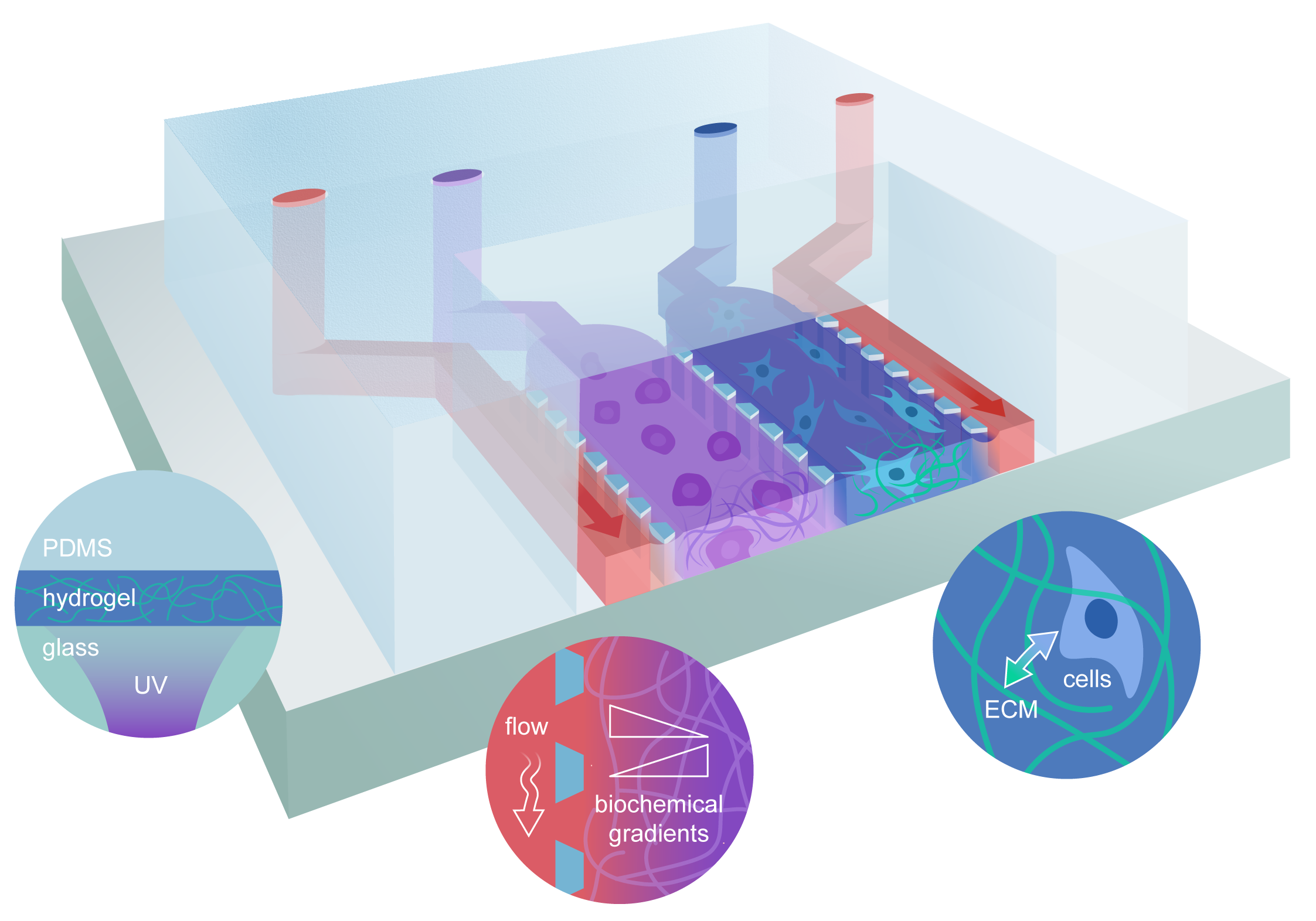
Figure 1
Schematic representation of the organ-on-chip setup used in this study, highlighting some of the factors impacting the mechanical microenvironment. The mechanical properties of the ECM-mimicking gels on-chip can be affected by (illustrated by the circles, from left to right): (i) factors intrinsic to the assembly procedure (such as light diffusion during photocrosslinking, shear stress during injection, temperature inhomogeneities, etc.); (ii) the effects of fluid flowing in the medium supply channels (physical, as in flow-induced shear stress, or biochemical, as in the opposite gradients of salts, nutrient and waste molecules); (iii) the bidirectional mechanical signaling between cells and the growth support.
Results
Model design and characterization: mechanical measurements on-chip reveal localized heterogeneity and the effect of OoC assembly on material properties.
The osteochondral interface was modelled with an OoC constituted by two chambers mimicking the bone and cartilage tissues. The chambers were separated by a discontinuous wall allowing close communication between the distinct compartments (Figure 2 and ref. 6). The cartilage tissue was modelled by embedding primary chondrocytes into gelatin methacrylate (GelMA) hydrogel. The same gel was doped with hydroxyapatite and populated with primary osteoblasts to replicate the bone compartment.
To assess the mechanical properties of the material alone, after integration into the system, we precisely mapped large areas of the cartilage matrix-mimicking and the bone matrix-mimicking materials on-chip. The use of the Pavone nanoindenter enabled access to the delaminated chip chambers, and the continuous mapping of the materials’ mechanical properties at high resolution. Localized mechanical characterization across an area spanning over the two chambers confirmed mechanically the compartmentalization of the hydrogels, with the bone-mimicking matrix displaying an overall higher Young’s modulus (18 kPa on average) than its cartilage-mimicking counterpart (16 kPa on average) (Figure 2). As expected, because of the local topography, the measurements performed along the separation wall axis resulted either in failed measurements (as indicated by blank datapoints in the figure) or in very high apparent Young’s modulus.
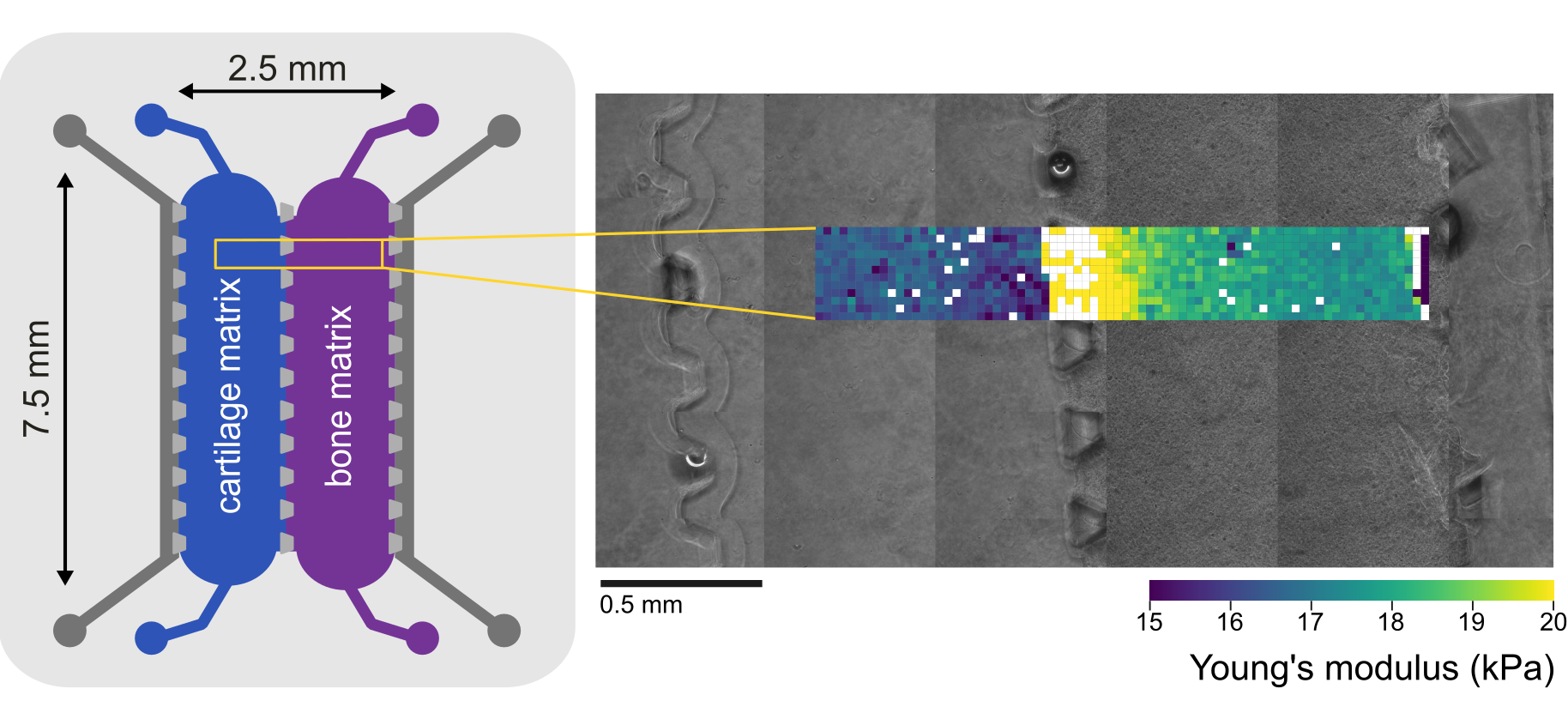
Figure 2
On-chip mapping of the mechanical properties of the cartilage matrix-mimicking and the bone matrix-mimicking materials shows localized variations across large hydrogel areas. Left: schematic representation of the osteochondral unit chip. Right: stitched image of a portion of the osteochondral unit and overlayed heatmap of measured Young’s modulus across the two chambers. Blank data points correspond to the position of the PDMS pillars separating the chambers.
The assembly of engineered tissues on-chip is a multistep process that has the potential to influence the final mechanical properties of the model. To control whether this effect was apparent in the osteochondral unit, we compared the mechanical properties of the cartilage- and bone-mimicking matrices when polymerized on-chip and off-chip (in well plates). Our findings show that the Young’s moduli of both materials differ between on-chip and off-chip conditions (where both materials have Young’s modulus values in the order of 6 kPa), suggesting that the experimental setup influences the materials’ mechanical properties (Figure 3). Multiple causes for the observed differences can be hypothesized, including, but not limited to, the effect of different gel states (e.g. due to local temperature differences before photocrosslinking), settling of particles before solidification, or differences in light intensity reaching the gel due to the different setups. Regardless of which factor specifically causes the measured difference, this finding indicates that the resulting mechanical milieu that cells experience on-chip cannot be directly inferred from off-chip measurements alone. An accurate control of the mechanical microenvironment on-chip requires, therefore, in situ measurement upon integration into OoC devices.

Figure 3
Young’s moduli of hydrogels on- and off-chip, as measured by indentation.
Mechanical phenotyping of the osteochondral interface-on-chip: inflammatory stimuli induce severe ECM modification
The possibility of accessing mechanical properties on-chip opens the way for monitoring their evolution in advanced models of physiopathological conditions. The evaluation of the cell culture environment in 3D models usually employs imaging techniques for the detection and visualization of the spatial organization of crucial components of the culturing matrix. These methods rely on the pre-selection of proteins of interest and on probe availability and, however highly informative, provide only an indirect measure of the changes in mechanical properties. In the context of osteoarthritis, it has been long identified how persistent inflammation activates ECM degradation and remodeling pathways intrinsic of the disease progression5.
To investigate the ability of the osteochondral unit in this study to replicate such changes, we assessed the mechanical properties of the two cell-laden compartments in the OoC under control (non-inflammatory) and inflammatory conditions. Our findings indicate that inflammatory stimuli can significantly affect the local mechanical properties of materials (Figure 4). The engineered tissues treated with the pro-inflammatory cocktail presented an overall lower Young’s modulus, indicating gel degradation (Figure 4). In particular, the cartilage-mimicking chamber was dramatically affected by inflammatory conditions leading to complete loss of gel integrity in a large portion of the chamber, as indicated by blank datapoints in the heatmap.
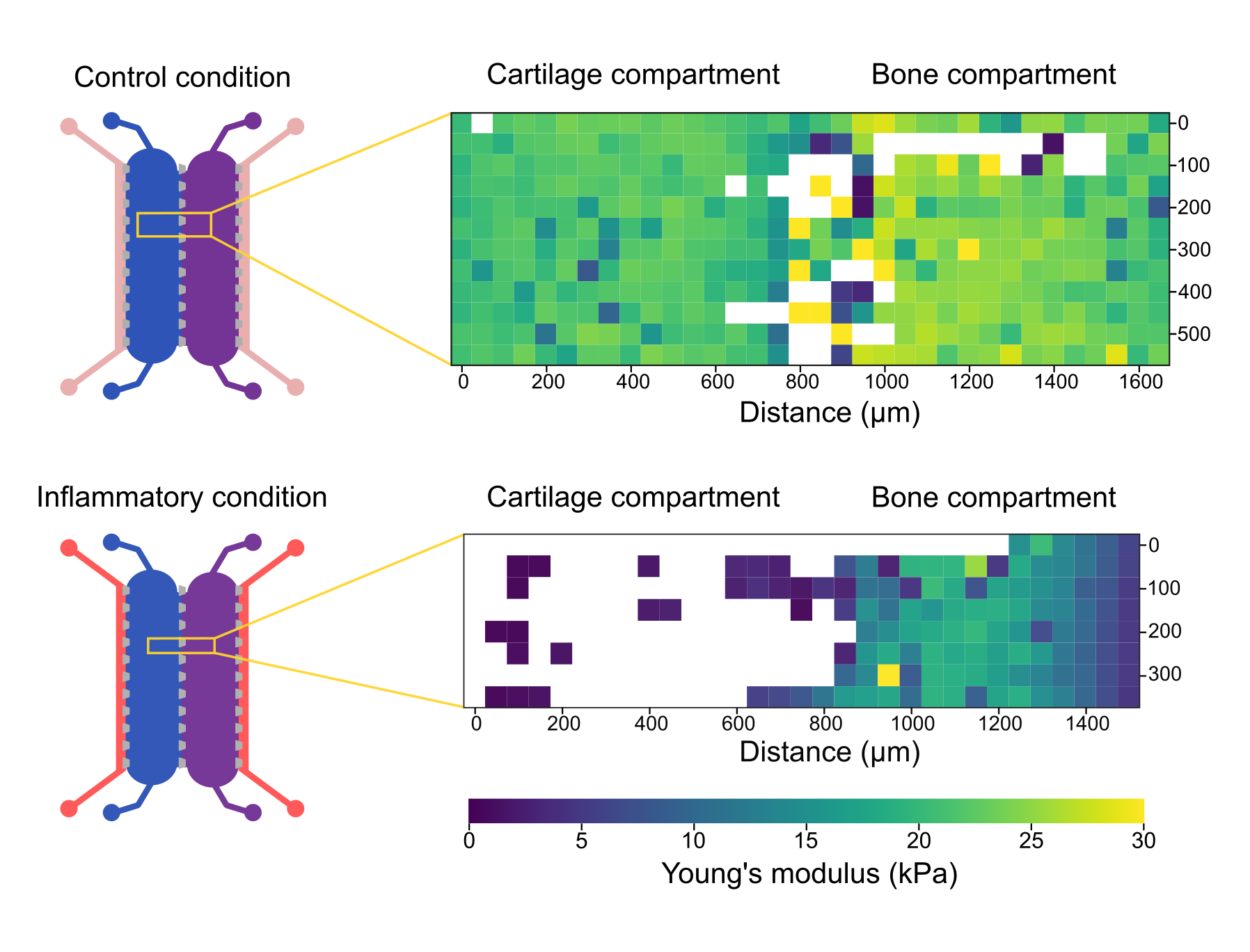
Figure 4
Pro-inflammatory conditions induce generalized softening of the cell-laden matrix, leading to loss of integrity on the cartilage compartment, as indicated by the blank datapoints.
Conclusion
Advancements in OoC technology are opening the way to the study of cellular and tissue function in dynamic controlled conditions in vitro. The resulting findings are of particular interest to shed light on physiopathological processes that cannot be studied by focusing on just one component (might that be cellular or extracellular) in isolation. An example is represented by cell-mediated remodeling of the mechanical integrity of the ECM, crucial in the progression of osteoarthritis.
To deliver on the promise that OoC represent, it is however important to adapt current assays to be compatible with the restrictions that the on-chip setup imposes. With this need in mind, the work presented in this application note applies the Pavone nanoindenter’s capability to mechanically characterize engineered tissues in situ. Our findings reveal that materials’ mechanical properties can differ significantly between off-chip, the most common testing setup, and on-chip, the actual environment that cells are exposed to. These differences, arising from the chip assembly procedure, can impact cellular behavior and experimental outcomes and should not therefore be overlooked in model design. Additionally, mapping the OoC chambers at high-resolution uncovered the effect of pro-inflammatory signals on the ECM mechanical properties at the local level offering a direct readout of matrix remodeling. By understanding and controlling mechanical properties on-chip, researchers can more accurately replicate physiological conditions and follow their response to pathological stimuli, improving the predictive capability of OoCs in studying human biology and disease in preclinical settings.
Methods
A brief description of the methods employed in this study is provided in this section to facilitate the data interpretation. For a more detailed description of the protocols and materials employed please refer to ref. 6.
OoC preparation
The osteochondral unit on-chip consisted of two individual chambers representing, respectively, the cartilage and the bone compartments of a joint. The two chambers were contained on a PDMS microfluidic platform on glass and divided by PDMS pillars. Both chambers were separated from a medium supply channel by PDMS pillars (Figure 2).
Two different formulations of engineered GelMA-based cell culture matrix were assembled in the OoC chambers. In short, 8% (w/v) of GelMA and 0.2% (w/v) of LAP (photoinitiator) were dissolved in PBS and, for the bone compartment, doped with 0.5% hydroxyapatite nanoparticles to mimic the mineralization of bone matrix. The hydrogel mix was then injected in the chip and polymerized by UV-induced crosslinking.
Cell culture and treatment
Human primary chondrocytes and human primary osteoblasts from surgical waste, sourced and cultured as described in ref. 6, were employed in the cartilage and bone compartments, respectively. For each chamber, 20000 cells were embedded in 5 µl of the unpolymerized matrix prior to the injection in the chip. During culture, osteoblasts or chondrocytes differentiation medium was flowed in the supply channel adjacent to the appropriate chamber.
Once assembled, the chips were maintained at 37°C in presence of 5% CO2 in a humidified incubator. To simulate inflammatory conditions, a cytokine cocktail containing IL-1β and TNF-α was added to the culture medium for a week.
Indentation assay
To access the hydrogel within the chip, the PDMS was manually separated from the glass, exposing the encapsulated hydrogel, which adhered either to the glass slide or to the PDMS chamber. For off-chip measurements, the hydrogels were crosslinked in a 48-well plate. Mechanical characterization was conducted with the Pavone nanoindenter (Optics11 Life). All measurements were performed on samples submerged in phosphate-buffered saline at 37°C.
Indentation tests were conducted using a probe with a 25 μm tip radius and 0.5 N/m stiffness. The local Young’s modulus of materials on-chip and off-chip was determined by fitting the indentation curves using a Hertzian model in the Optics11 Life’s DataViewer software.
Acknowledgments
Microfluidic devices were kindly supplied by Dr. Francisco Conceição and Dr. Liliana Moreira Teixeira
from the Bioengineering Technologies department at the University of Twente.
References
1. Muncie JM, et al. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Current Topics in Developmental Biology vol. 130 (2018): 1-37. doi:10.1016/bs.ctdb.2018.02.002.
2. Kutluk H, et al. Integration of Extracellular Matrices into Organ-on-Chip Systems. Advanced Healthcare Materials vol. 12,20 (2023): e2203256. doi:10.1002/adhm.202203256.
3. Thompson CL, et al. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Frontiers in Bioengineering and Biotechnology vol. 8 602646. 10 Dec. 2020, doi:10.3389/fbioe.2020.602646.
4. Urciuolo F, et al. In vitro strategies for mimicking dynamic cell-ECM reciprocity in 3D culture models. Frontiers in Bioengineering and Biotechnology vol. 11 1197075. 26 Jun. 2023, doi:10.3389/fbioe.2023.1197075.
5. Yao Q, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduction and Targeted Therapy vol. 8,1 56. 3 Feb. 2023, doi:10.1038/s41392-023-01330-w.
6. Conceição F, et al. Sex-stratified osteochondral organ-on-chip model reveals sex-specific responses to inflammatory stimulation. Materials Today Bio vol. 32 101728. 2 Apr. 2025, doi:10.1016/j.mtbio.2025.101728.
7. Leung CM, et al. A guide to the organ-on-a-chip. Nat Rev Methods Primers vol. 2,33. 12 May 2020, doi:10.1038/s43586-022-00118-6.
8. Tajeddin A, et al. Design and Fabrication of Organ-on-Chips: Promises and Challenges. Micromachines vol. 12,12 1443. 25 Nov. 2021, doi:10.3390/mi12121443.


