Implications for fibrosis research and drug discovery
by Optics11 Life
© 2024 Optics11 Life B.V.
Cardiac fibrosis, a consequence of myocardial insults, manifests as an abnormal accumulation of extracellular materials, which compromise cardiomyocyte composition, nanostructure, and mechanics, ultimately leading to heart failure. However, suitable analyses are lacking to study cardiac fibrosis mechanisms and develop effective therapies. This application note explores alterations in the mechanical properties and composition of cardiac-specific decellularized matrices under pro-fibrotic conditions, allowing the study of critical biological and mechanical changes during myocardial fibrosis.
Introduction
Cardiac fibrosis is a pathological wound healing caused by an excessive accumulation of fibrous connective tissue within the heart. This process disrupts the heart’s architecture and function, impairing cardiac performance and causing life-threatening complications1. In the event of myocardial injury or stress, the body initiates a repair process that involves the formation of scar tissue primarily composed of collagen and other extracellular matrix (ECM) proteins2. While wound healing is necessary to maintain the heart’s structural integrity, excessive or uncontrolled ECM deposition can impair cardiac function by stiffening the myocardium3, disrupting electrical conduction4, and interfering with the relaxation and contraction of the heart chambers5.
However, one of the primary challenges in addressing cardiac fibrosis lies in the complexity of its pathophysiology and the lack of suitable methods for studying its mechanisms and developing effective therapies. Cardiac fibrosis models often focus on individual proteins that provide a limited view of disease development. In contrast, measuring mechanical properties altered by fibrosis can serve as a tool for identifying and characterizing disease status and progression and facilitating drug discovery.
This application investigates changes in the mechanical properties and composition of cardiac-specific decellularized matrices (dECMs) derived from human induced pluripotent stem cell-derived cardiac fibroblasts under pro-fibrotic conditions. These dECMs serve as substrates for cardiomyocyte cultures, facilitating the study of critical biological and mechanical alterations at the cellular level induced by fibrotic dECM. This study was conducted in collaboration with the International Clinical Research Center (ICRC – St Anne’s University Hospital Brno and the School of Cardiovascular and Metabolic Medicine and Sciences – King’s College London), and published in Translational Research6.
Methods
Generation and characterization of fibrotic dECMs
Human dECMs were obtained from cardiac fibroblasts differentiated from human induced pluripotent stem cells (iPSCs). Fibrotic dECMs were generated by manipulating anti- and pro-fibrotic pathways with growth factors such as basic fibroblast growth factor (bFGF) and transforming growth factor beta 1 (TGF-β1). To characterize the dECMs, confocal microscopy, mass spectrometry, and nanoindentation techniques were employed to reveal their composition and mechanical properties. The aim was to elucidate how pathological stimuli-induced remodeling of cardiac ECM influences myocardial microtopography, stiffness, and viscoelasticity.
Mechanical property characterization
Nanoindentation was conducted with the Pavone nanoindenter to measure micro-mechanic signatures of pro-fibrotic, control, and anti-fibrotic dECMs. All measurements were performed at room temperature with samples fully hydrated in PBS. A probe with a 9.5 µm tip radius and stiffness of 0.49 N/m, as well as a tip with a 24 µm radius and stiffness of 0.018 N/m, were utilized, adjusted according to sample stiffness and topography. To ensure accurate measurements, samples with inhomogeneities in ECM surface coverage were indented only in areas visually confirmed to contain ECM, avoiding areas without ECM coverage.
Young’s modulus was determined for each condition by analyzing indentation curves (from contact to 500 nm indentation depth) and fitting them to the Hertzian model. Surface topography was assessed by comparing relative z-positions, with z = 0 µm representing the well plate surface. Dynamic measurements were performed to investigate frequency-dependent viscoelastic behavior.
Results
ECM structural and basal components
The leading indicator of fibrotic conditions is the abnormal accumulation of ECM proteins. Confocal microscopy analysis revealed a thin layer of ECM proteins in anti-fibrotic dECMs and a dense honeycomb of intertwined fibers in pro-fibrotic dECMs. Further characterization of the dECMs using antibodies targeting ECM components (collagen I and IV, fibronectin, ED-A and ED-B fibronectin isoforms, and laminin) confirmed enhanced deposition of ECM components in the pro-fibrotic condition compared to control and anti-fibrotic conditions (Figure 1).
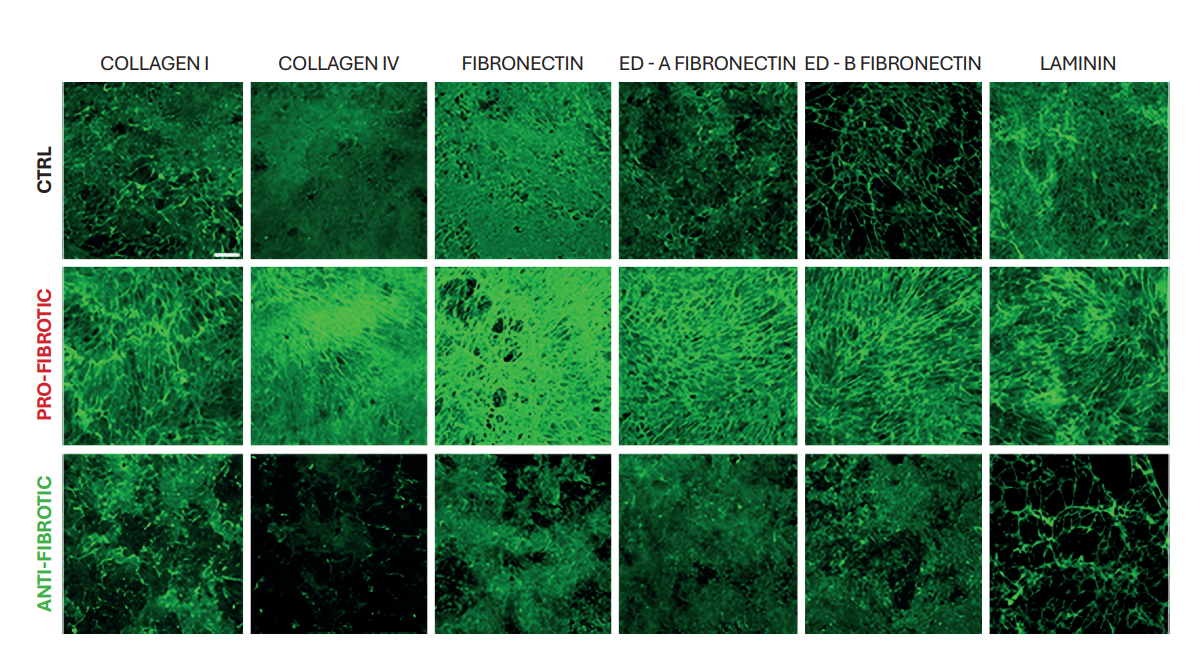
Figure 1
ECM remodeling. Representative confocal images of control (ctrl), pro-fibrotic, and anti-fibrotic dECMs stained for collagen I, collagen IV, fibronectin, ED-A fibronectin, and ED-B fibronectin, ED-A fibronectin and ED-B fibronectin isoforms, and laminin (AF488, green) (Scale bar: 50 µm).
Note. Adapted from “Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis”, Niro, F, 2024, Translational Research. 2024; 273; 58–77. doi: 10.1016/j.trsl.2024.07.003. 1931-5244/©2024 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY license.
Differences in protein composition
The variations in dECM architecture stem from differences in protein composition, confirmed through mass spectrometry analysis. Among the identified components, 32 proteins exhibited upregulation in the pro-fibrotic ECM. These included structural ECM proteins such as collagens (COL1 and COL4) and glycoproteins (FBLN5, POSTN, and FN), and proteins associated with ECM elasticity, including SPARC and ELN. Heatmap representation showed the difference in the matrix composition within the control, anti-fibrotic, and pro-fibrotic conditions (Figure 2).
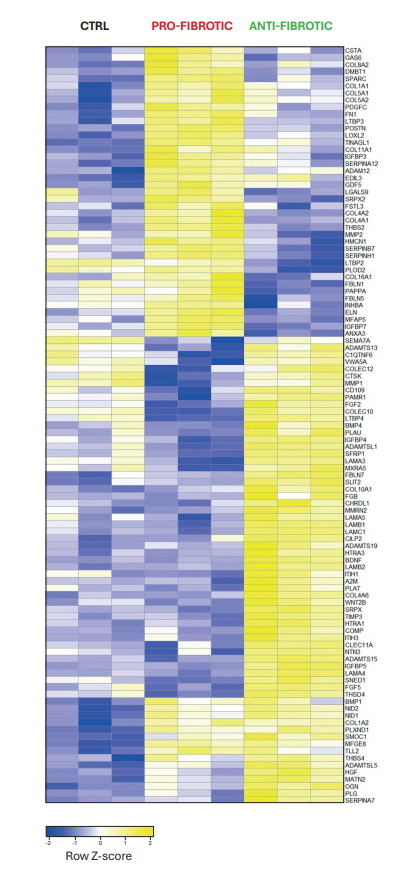
Figure 2
Heatmap of the most representative differentially regulated ECM proteins in control (ctrl), pro-fibrotic, and anti-fibrotic dECM. The values are normalized and displayed on the heatmap as Row Z-score values (-2 ≤ Z-score ≤ 2).
Note. Adapted from “Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis”, Niro, F, 2024, Translational Research. 2024; 273; 58–77. doi: 10.1016/j.trsl.2024.07.003. 1931-5244/©2024 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY license.
ECM mechanical properties
A high throughput nanoindentation strategy was adopted to evaluate the micromechanical properties of dECMs. Consistent with the pronounced variances observed in the structure and protein composition of pro-fibrotic ECM, ECM mechanical properties exhibited distinct signatures. In terms of microtopography, pro-fibrotic dECM presented irregular surfaces with increased thickness, while control dECM had a smoother surface, and anti-fibrotic dECM appeared flatter and thinner (Figure 3A). Notably, the stiffness of dECMs fell within the kilopascal (kPa) range, aligning with the physiological Young’s modulus values reported in the myocardium. The findings indicated a significant increase in Young’s modulus in pro-fibrotic dECM compared to control and anti-fibrotic dECM (Figure 3B). Moreover, local microrheological measurements revealed pro-fibrotic dECM forming a stiffer and more elastic network with minimal changes at higher frequencies. In contrast, the control dECM displayed significant viscoelastic changes with increasing frequencies (Figure 3C).
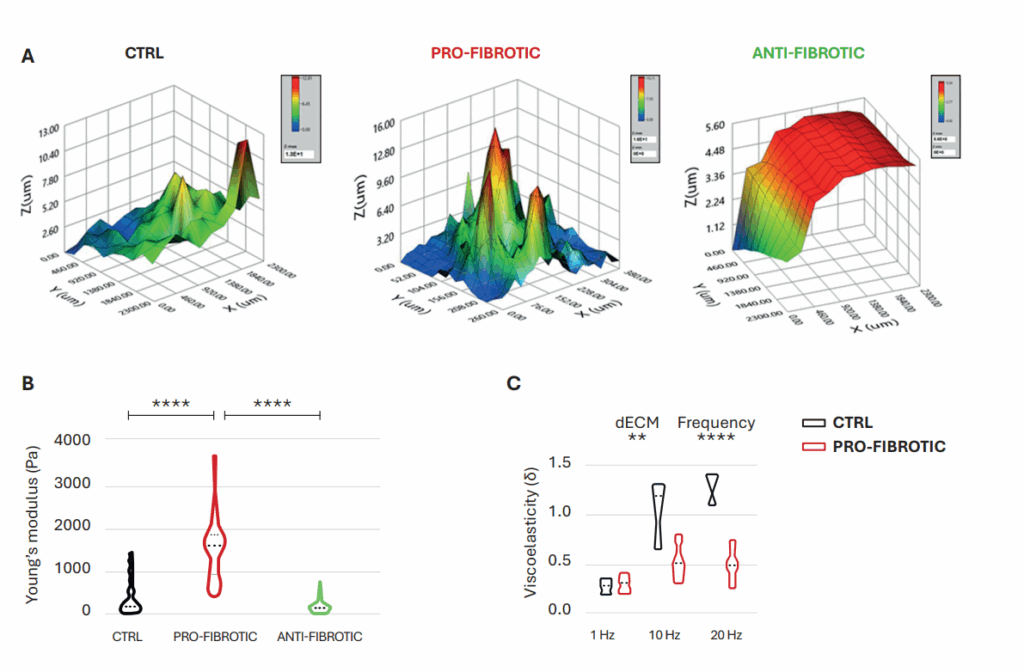
Figure 3
Mechanical properties of fibrotic and non-fibrotic dECM measured by Pavone. (A) Topographic maps of the dECM’s surface. (B) Violin plot representation of Young’s modulus analysis of the dECMs. (C) Dynamic measurements of viscoelastic modulus indented at a frequency of 1–10–20 Hz expressed as the viscous to elastic properties ratio of the material (tan δ).
Note. Adapted from “Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis”, Niro, F, 2024, Translational Research. 2024; 273; 58–77. doi: 10.1016/j.trsl.2024.07.003. 1931-5244/©2024 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY license.
Conclusion
This study explored the changes in decellularized matrices obtained from iPSCs under pro-fibrotic conditions. Here, we examined variations in the mechanical properties of in vitro-generated ECM through the high-throughput technology Pavone, which facilitated the association of local micromechanics with the topographic map of the matrices. The findings underscored the significance of ECM remodeling in driving cardiac dysfunction by revealing altered elastic and viscoelastic properties in the pro-fibrotic ECM, corresponding to notable variations in its structure and protein composition.
Therefore, mechanical measurements can be a quantitative functional biomarker to evaluate fibrotic conditions. This approach offers a broader perspective than examining individual fibrotic proteins and components, which offers a limited view of disease development. Moreover, it can be used interchangeably with other techniques, streamlining workflow. The interconnected findings provide a basis for improved in vitro models and targets for drug development to mitigate cardiac fibrosis and its associated complications.
Acknowledgments
We thank Dr. Giancarlo Forte and his research team at the International Clinical Research Center (ICRC) – St Anne’s University Hospital Brno and the School of Cardiovascular and Metabolic Medicine and Sciences – King’s College London for their invaluable scientific insights and expertise in developing the experiments described in this application note.
Disclaimer
All materials have been reproduced in accordance with the Creative Commons BY (CC-BY) license with appropriate credit.
References
1 Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells. 2021, 10, 1716. doi:10.3390/cells10071716.
2 Thomas TP, Grisanti LA. The Dynamic Interplay Between Cardiac Inflammation and Fibrosis. Front Physiol. 2020 Sep 15; 11:529075. doi:10.3389/fphys.2020.529075.
3 Lizardi JCV, et al. A guide for assessment of myocardial stiffness in health and disease. Nat Cardiovasc Res. 2022; 1:8–22. doi:10.1038/s44161-021-00007-3.
4 Verheule S, Schotten U. Electrophysiological Consequences of Cardiac Fibrosis. Cells. 2021 Nov 18; 10(11):3220. doi:10.3390/cells10113220.
5 Liu H, et al. Dynamic and static biomechanical traits of cardiac fibrosis. Front Bioeng Biotechnol. 2022 Oct 31; 10:1042030. doi:10.3389/fbioe.2022.1042030.
6 Niro F, et al. Fibrotic extracellular matrix impacts cardiomyocyte phenotype and function in an iPSC-derived isogenic model of cardiac fibrosis. Translational Research. 2024; 273:58–77. doi:10.1016/j.trsl.2024.07.003.


