By Optics11 Life
© 2023 Optics11 Life B.V.
Explore the impact of mechanobiology on fibrotic diseases with the advanced Pavone
Mechanical forces are essential to fibrosis development and progression. In this sense, cell mechanics is a potential label-free biomarker for fibrotic diseases. Here, we introduce the highthroughput mechanical screening platform Pavone for thoroughly characterizing fibrotic tissues and developing innovative therapies.
Introduction
Mechanical forces are essential to the development and progression of fibrosis1. Cells and tissues behave like viscoelastic matter that exhibits viscous and elastic characteristics when subjected to deformation. Changes in their mechanical properties, such as stiffness and viscosity, affect their behavior and play a significant role in tissue injury, repair, and fibrosis2. Recent evidence confirms that changes in the mechanical properties of the extracellular matrix (ECM), such as matrix stiffness, play a crucial role in developing fibrosis by mechano-activation of fibroblasts, which remodel normal tissue into non-functional fibrotic tissue. Once activated, fibroblasts produce and deposit excessive amounts of ECM components, leading to tissue fibrosis3.
For example, the stiffness of the skin, lung, and liver increases from 0.5 to 1 kPa in homeostasis up to 25–100 kPa in animal models of fibrosis4. Given the evidence of ECM’s contribution to disease progression, investigating the processes underlying perturbation of homeostasis, the changes in biomechanical ECM properties, and the resulting cell–ECM interactions will allow researchers to identify early signs of fibrotic disease and novel therapeutic approaches.
However, traditional biological studies overlook the impact of mechanical forces on fibrosis development. Besides, conventional methods for the mechanical characterization of cells and tissues are complex, as they require time-consuming sample preparations, which may lead to losing some of their native mechanical properties. To address the unfulfilled needs in fibrosis, we present the Pavone nanoindenter as a novel tool to characterize the mechanics of cells, tissues, spheroids, organoids, and biomaterials. This innovative approach monitors the mechanical environments to which cells are exposed in health and disease, clarifies the mechanical responses caused by disturbances, and unlocks innovative therapeutic strategies. In contrast to most classical instruments, Pavone non-destructively characterizes a smaller sample volume and allows a more realistic profile of its nanoscale mechanical properties.
Optics11 Life novel technology
Pavone provides a mechanical screening platform for novel applications, such as disease modeling, drug screening and delivery, regenerative therapies, genetic medicine, and diagnosis of diseases. For instance, Pavone monitors the mechanical environments in healthy, injured, repaired, and fibrotic tissues5. By measuring changes in the mechanical properties of fibrotic tissues over time, Pavone assesses the effectiveness of antifibrotic drugs in reducing stiffness and restoring normal tissue function6,7.
Additionally, Pavone supports advanced 3D in vitro systems, which more accurately mimic the fibrotic microenvironment. These include scaffold- or hydrogel-based structures8,9,10, spheroids11, and organoids12,13. By creating 3D models of cells and surrounding tissues, researchers simulate living tissue’s mechanical and biochemical properties to optimize therapeutic delivery methods and discover potential therapies for fibrotic diseases.
Pavone features a unique combination of automated indentation mapping, bright-field and fluorescence imaging, and environmental control. Compatible with up to 2 × 96-well plates, Pavone is the only instrument to measure soft materials in well plates for large and extended living-sample experiments. Compared to most conventional methods, Pavone stands out by providing simple and easy workflows that do not involve specialized operators or extensive training. Therefore, it minimizes equipment requirements and reduces processing times and costs.
Mechanical characterisation of ECM hydrogels
Fibrotic lung diseases result from alterations in the ECM’s composition and abundance that affect the lung’s mechanical properties. Therefore, the mechanical properties of the fibrotic ECM are often mimicked in vitro using hydrogels. ECM hydrogels, made from native decellularized tissue, retain most of the native ECM composition and generally reproduce the mechanical properties of the parent tissue14,15.
Here, we investigated the mechanical properties of a fibrotic microenvironment of lung-derived ECM hydrogels using Pavone. To generate hydrogels, fibrotic lung tissues were decellularized, lyophilized, ground into powder, porcine pepsin–solubilized, buffered with PBS, and gelled at 37°C. We used two lung-derived ECM hydrogels in duplicates of similar composition and different fibrosis levels (ECM hydrogels A and B). Hydrogels’ topographical and mechanical properties were assessed with a probe with a stiffness of 0.5 N/m and a tip radius of 25.0 μm.
First, we employed Young’s modulus to measure the stiffness of the lung-derived ECM hydrogel. Our findings revealed that ECM hydrogels A exhibited higher stiffness than ECM hydrogels B (364 ± 151 Pa and 297 ± 68.1 Pa for ECM hydrogels A, and 21.7 ± 6.68 Pa and 20.3 ± 7.66 Pa for ECM hydrogels B) (Figure 1), confirming a different degree of fibrosis.
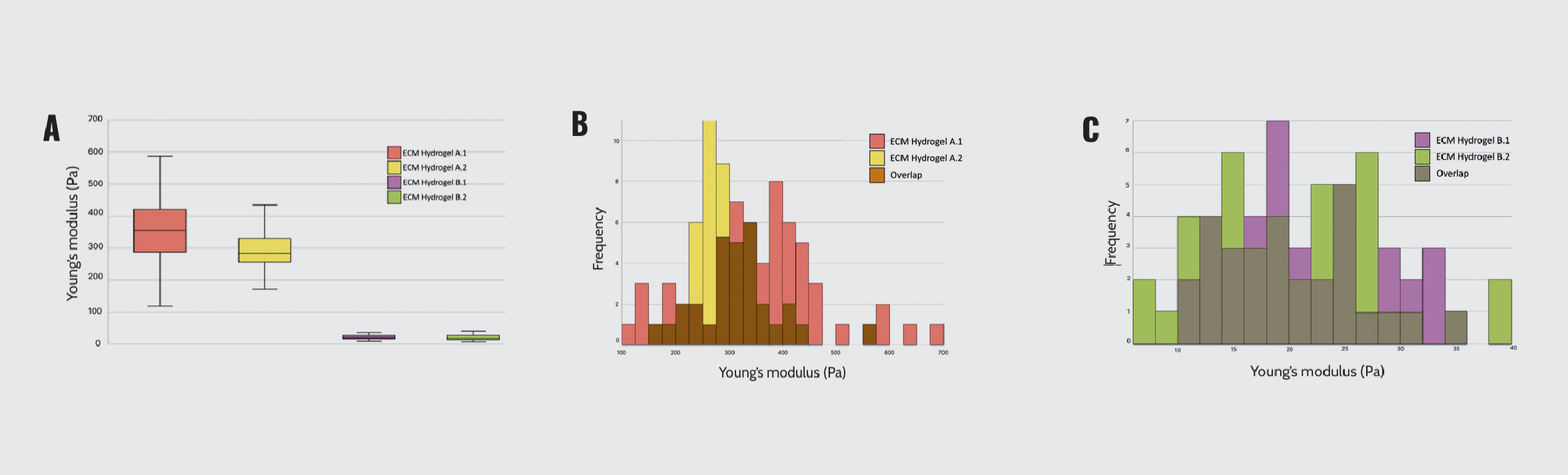
Mechanical characterization of lung-derived ECM hydrogels. (A) Young’s modulus of ECM hydrogels A and B. (B) Mechanical profile of ECM hydrogel A duplicates. (C) Mechanical profile of ECM hydrogel B duplicates.
Then, we focused on ECM hydrogels A and its surface plot of Young’s modulus and topography (Figure 2). Young’s modulus ranged from 149.22 to 575.57 Pa, with a mean value of 362.39 Pa (Figure 2A). Additionally, the topography map indicated a range of heights up to 29.04 μm, with a mean of 14.52 μm (Figure 2B).
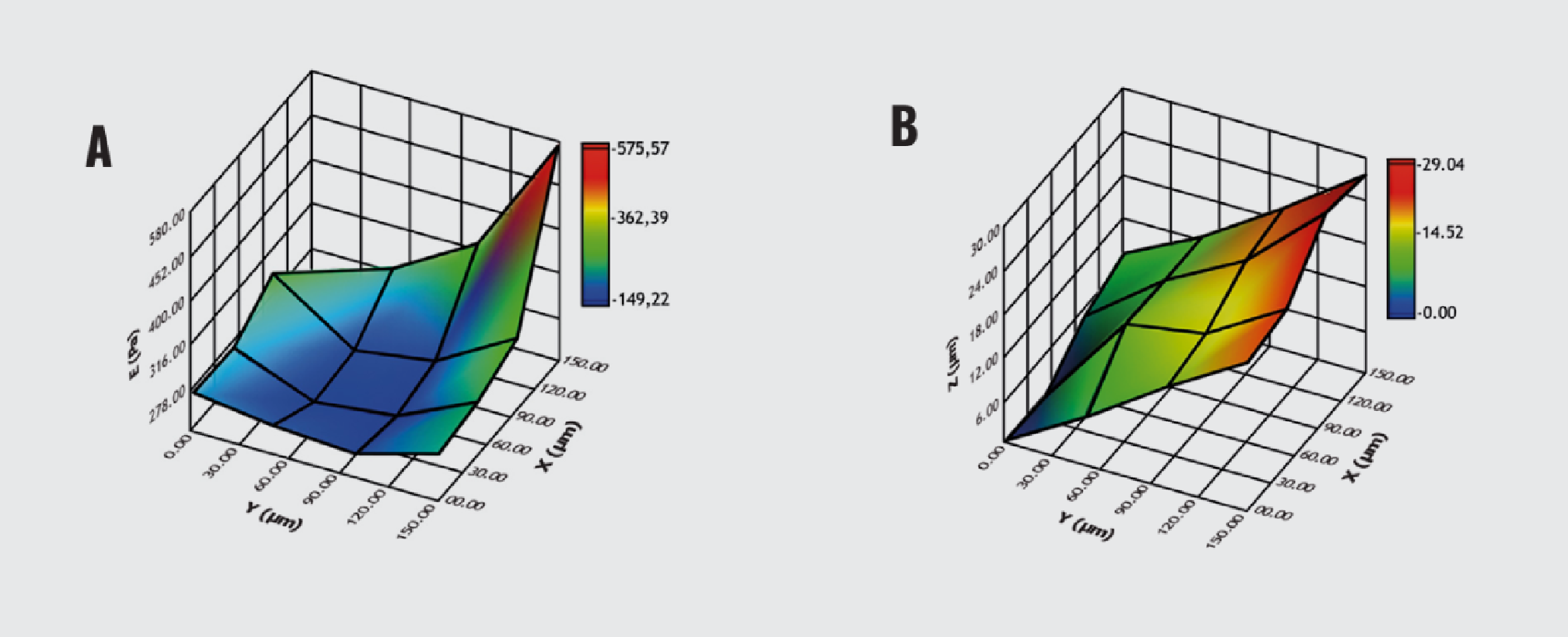
3D map of ECM hydrogel. (A) Surface plot of Young’s modulus. (B) Surface plot of topography.
Understanding the association between mechanical features and clinical manifestations in fibrotic lung diseases will contribute to earlier diagnosis and novel therapies. In this sense, Pavone is an innovative tool for investigating biomechanical alterations in fibrotic ECM.
Conclusion
Fibrosis causes progressive deterioration of tissue mechanical behavior, making mechanics a potential label-free biomarker for disease development. Developing studies on tissue mechanics leads to new targets for fibrosis treatments. In this sense, Pavone is a promising technology for screening-scale research on fibrosis. Translating tissue mechanics into clinical and therapeutic interventions will enable new approaches to treating fibrotic tissue remodeling.
References
- Tschumperlin D. J, Ligresti G, Hilscher M. B, Shah V. H. Mechanosensing and fibrosis. J Clin Invest. 2018;128(1):74–84. doi: 10.1172/JCI93561.
- Mierke C. T. Viscoelasticity, like forces, plays a role in mechanotransduction. Front Cell Dev Biol. 2022;10:789841. doi: 10.3389/fcell.2022.789841.
- Cox T. R, Erler J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4(2):165–78. doi: 10.1242/dmm.004077.
- Tschumperlin D. J, Lagares D. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacol Ther. 2020;212:107575. doi: 10.1016/j.pharmthera.2020.107575.
- Akinbote A, Beltran-Sastre V, Cherubini M, Visone R, Hajal C, Cobanoglu D, Haase K. Classical and non-classical fibrosis phenotypes are revealed by lung- and cardiac-like microvascular tissues on-chip. Front Physiol. 2021;12:735915. doi: 10.3389/fphys.2021.735915.
- Hoffmann M, Kant T. A, Emig R, Rausch J. S. E, Newe M, Schubert M, Kunzel K, Winter L, Klapproth E, Peyronnet R, Ravens U, El-Armouche A, Kunzel S. R. Repurposing mesalazine against cardiac fibrosis in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(3):533–543. doi: 10.1007/s00210-020-01998-9.
- Kunzel S. R, Rausch J. S. E, Schaffer C, Hoffmann M, Kunzel K, Klapproth E, Kant T, Herzog N, Kupper J. H, Lorenz K, Dudek S, Emig R, Ravens U, Rog-Zielinska E. A, Peyronnet R, El-Armouche A. Modeling atrial fibrosis in vitro – generation and characterization of a novel human atrial fibroblast cell line. FEBS Open Bio. 2020;10(7):1210–1218. doi: 10.1002/2211-5463.12896.
- Chen G, Deng Y, Xia B, Lv Y. In situ regulation and mechanisms of 3D matrix stiffness on the activation and reversion of hepatic stellate cells. Adv Healthc Mater. 2022; e2202560. doi: 10.1002/adhm.202202560.
- Hui E, Moretti L, Barker T. H, Caliari S. R. The combined influence of viscoelastic and adhesive cues on fibroblast spreading and focal adhesion organization. Cell Mol Bioeng. 2021;14(5):427–440. doi: 10.1007/s12195-021-00672-1.
- Kumar M, Toprakhisar B, Van Haele M, Antoranz A, Boon R, Chesnais F, De Smedt J, Tricot T, Idoype T. I, Canella M, Tilliole P, De Boeck J, Bajaj M, Ranga A, Bosisio F. M, Roskams T, van Grunsven L. A, Verfaillie C. M. A fully defined matrix to support a pluripotent stem cell–derived multicell liver steatohepatitis and fibrosis model. Biomaterials. 2021;276:121006. doi: 10.1016/j.biomaterials.2021.121006.
- Liu Y, Yang X, Donj J, Rongchen H, Huang F, Zou X, Liu C, Peng Z. 3D biomimetic tumor microenvironment of HCC to visualize the intercellular crosstalk between hepatocytes, hepatic stellate cells, and cancer cells. 2022. doi: 10.2139/ssrn.4246793.
- van Tienderen G. S, Rosmark O, Lieshout R, Willemse J, de Weijer F, Elowsson Rendin L, Westergren-Thorsson G, Doukas M, Groot Koerkamp B, van Royen M. E, van der Laan L. J, Verstegen M. M. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023;158:115–131. doi: 10.1016/j.actbio.2022.11.038.
- Willemse J, van Tienderen G, van Hengel E, Schurink I, van der Ven D, Kan Y, de Ruiter P, Rosmark O, Westergren-Thorsson G, Schneeberger K, van der Eerden B, Roest H, Spee B, van der Laan L, de Jonge J, Verstegen M. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022;284:121473. doi: 10.1016/j.biomaterials.2022.121473.
- Nizamoglu M, de Hilster R. H. J, Zhao F, Sharma P. K, Borghuis T, Harmsen M. C, Burgess J. K. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023;158:115–131. doi: 10.1016/j.actbio.2022.11.038.
- de Hilster R. H. J, Sharma P. K, Jonker M. R, White E. S, Gercama E. A, Roobeek M, Timens W, Harmsen M. C, Hylkema M. N, Burgess J. K. Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L698–L704. doi: 10.1152/ajplung.00451.2019.

