Measuring the mechanobiology of organoids as a label-free biomarker
© 2023 Optics11 Life B.V
Mechanical forces in organoids act as functional read-out parameters, influencing their phenotype, morphology, and function. However, the mechanical characterization of organoids can be challenging, involving fixation methods that compromise their native properties. This application note describes the non-destructive characterization of live organoids in a user-friendly workflow using the Pavone mechanical screening platform.
Introduction
Organoids are 3D tissue cultures with similar physiological structure and function to living organs. In contrast to traditional 2D cell cultures, organoids offer a more accurate representation of the organ environment in vitro1,2. As organoids provide a framework to study mechanical aspects in 3D, integrating mechanobiology into organoid technology can create more physiologically relevant in vitro models. These mechanical properties serve as functional read-out parameters, revealing how mechanical forces determine the phenotype, morphology, and function of organoids3.
However, assessing organoids’ mechanical properties can be complex and time-consuming, often involving fixation methods that compromise their native mechanical features. Pavone offers a solution by streamlining organoid assessments: it minimizes equipment requirements, processing times, and costs by providing simple and easy workflows that do not require specialized operators or extensive training. Additionally, Pavone non-destructively characterizes live organoids, providing a more comprehensive profile of their mechanical fingerprint.
This technical note describes the sample preparation and mechanical screening of organoids using Pavone – a novel mechanical screening platform for thoroughly characterizing organoids.
Procedure
A. Sample preparation
The nanoindentation technology of Pavone allows mechanical probing of biological samples under standard culture conditions. However, the growth and differentiation of organoids often involve embedding them within extracellular matrix scaffolds such as Matrigel©, which can compromise sample accessibility and decrease measurement accuracy. To prevent these issues, it is essential to employ a validated method to extract organoids from their 3D support, such as Corning’s cell recovery solution and protocol4.
Furthermore, immobilizing organoids on a substrate might be necessary to minimize lateral movements during indentation. Various methods have been developed for this purpose5,6,7. For an effective and simple method of sample immobilization, transfer the organoids to a well plate previously coated with an extracellular matrix protein (e.g., collagen or laminin) or polyethyleneimine solution. Determine the immobilization substrate based on the specific characteristics of the samples.
B. Mechanical screening
Pavone provides mechanical measurements recorded with cantilever-based force sensors, e.g., probes (Figure 1A). The probes feature a broad range of stiffnesses and dimensions. However, selecting a probe with an appropriate tip size and spring constant can significantly enhance the quality and spatial resolution of the measurements. In our experiment, a probe with a stiffness of 0.5 N/m and a tip radius between 10 and 50 μm proved ideal for the mechanical characterization of organoids.
The matrix scan function allows the mapping of organoids’ mechanical properties at the nanoscale by indenting different locations. Here, we adjusted the distance between indentations and the total size of the scanned grid with Pavone software. The selection of these parameters should align with the desired resolution and the total dimension of the organoids.
The Pavone software supports different indentation modalities for the mechanical characterization of the organoid. In our experiment, different modes of operation and indentation profiles enabled the measurement of specific mechanical properties, such as viscoelasticity and Young’s modulus. To obtain a highly resolved mechanical map of the organoids’ stiffness in a time-effective manner, we set up the matrix scan using the peak load poking (PLP) mode. To rapidly screen a large number of organoids, it is possible to configure an automated indentation sequence, allowing the acquisition of a high volume of data with minimal operator engagement.
C. Data analysis
The DataViewer software assists in easily fitting the load–indentation curves obtained with Pavone to extract Young’s modulus of the probed area. Additionally, it automatically generates a representation of the results as a histogram of values distribution (Figure 1B) or as a spatially resolved heat map (Figure 1C).
In parallel, the software allows the sample’s topography in 3D (Figure 1D) or 2D (Figure 1E), showing the immediate concurrent visualization of the sample stiffness and morphology.
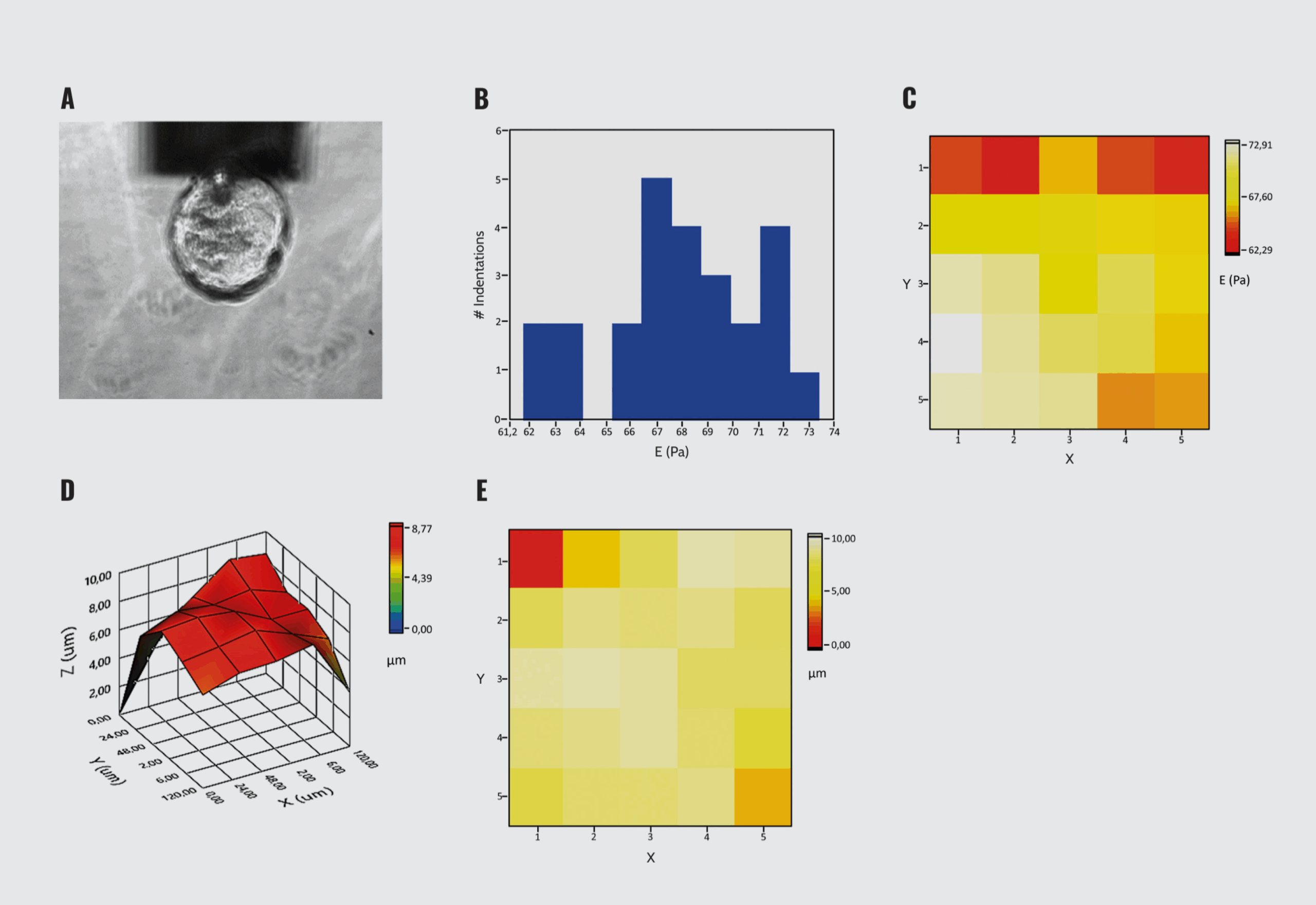
Mechanical characterization of organoids with Pavone. (A) Indentation of organoids. (B) Histogram of Young’s modulus distribution. (C) 2D Young’s modulus heat map. (D) 3D surface topography. (E) 2D surface plot. The graphical representations are generated by the DataViewer.
Conclusion
In this technical note, we described the use of Pavone to facilitate the mechanical properties assessment of organoids. We have demonstrated:
- Pavone performs automated indentation of live organoids without compromising their mechanical integrity.
- Pavone requires minimal sample preparation for the mechanical characterization of live organoids.
- Pavone enables mechanical screening of large sample sets within a short timeframe.
References
- Kratochvil M. J, et al. Engineered materials for organoid systems. Nat Rev Mater. 2019 Sep;4(9):606–622.
- Bayir E, et al. Mechanobiology of cells and cell systems, such as organoids. Biophys Rev. 2019 Oct;11(5):721–728.
- Dahl-Jensen S, Grapin-Botton A. The physics of organoids: a biophysical approach to understanding organogenesis. Development. 2017 Mar 15;144(6):946–951.
- Extraction of three-dimensional structures from Corning® Matrigel® matrix – guidelines for use (https://www.corning.com/catalog/cls/documents/protocols/CLS-AN-528.pdf).
- Ryu H, et al. Transparent, compliant 3D mesostructures for precise evaluation of mechanical characteristics of organoids. Adv Mater. 2021 Jun;33(25):e2100026.
- Heuer R. A, et al. Three-dimensional otic neuronal progenitor spheroids derived from human embryonic stem cells. Tissue Eng Part A. 2021 Feb;27(3–4):256–269.
- Feijao T, et al. Engineering injectable vascularized tissues from the bottom-up: dynamics of in-gel extraspheroid dermal tissue assembly. Biomaterials. 2021 Dec;279:121222.

