by Optics11 Life
© 2023 Optics11 Life B.V.
Discover mechanical forces with the Pavone: a novel label-free biomarker for tumors
Mechanical properties in the tumor microenvironment play a crucial role in tumor growth, proliferation, and drug resistance. Mechanical changes in the tumoral tissue and its surroundings support tumor progression and metastasis by altering the metabolism and behavior of cancer cells and the associated stromal cells. In this sense, cell mechanics is a potential label-free biomarker for cancer development. Here, we introduce Pavone – a high-throughput mechanical screening platform for thoroughly characterizing a bladder tumor model.
Introduction
Tumor development is a complex, dynamic, continuous, and progressive process affected by the mechanical properties of cancer cells and their environment. Throughout tumor formation, the extracellular matrix (ECM) undergoes intricate mechanical alterations that impact and facilitate tumor progression, particularly during invasion and metastasis formation¹,². While cancer cells are generally softer than healthy cells, tumors tend to be stiffer and more heterogeneous than surrounding tissues due to matrix stiffening linked to fibrosis¹,³.
The inherent complexity and variability of tumor growth dynamics and metastasis timing make early cancer detection challenging. Although most cancers’ genetic and biochemical drivers are widely recognized, biophysical stimuli also contribute to tumor growth. Therefore, advanced mechanical characterization tools may deepen comprehension of cancer morphology and mechanics and their role in disease progression.
However, conventional methods for mechanically characterizing tumors, such as an atomic force microscope (AFM), are complex and time-consuming. They often involve sample preparations that compromise the tumor’s native mechanical properties. In response to the existing gaps in cancer research, we present the Pavone as a novel instrument to mechanically characterize cells, tissues, spheroids, organoids, and biomaterials in a non-destructive manner. This innovative approach can assay the mechanical environments cells experience in health and disease conditions and elucidate the mechanical responses triggered by disturbances, with the potential to unlock innovative therapeutic strategies.
Optics11 Life novel technology
Pavone provides a mechanical screening platform for novel research applications, such as disease modeling, drug screening and delivery, regenerative medicine, tissue engineering, and diagnosis of diseases. In particular, it can identify the mechanical difference between healthy and malignant cells and monitor the mechanical environments in cancer progression⁴. By measuring changes in tumor mechanical properties over time, Pavone assesses the effectiveness of anticancer drugs in reducing stiffness, restoring normal tissue function, and potentially overcoming tumor drug resistance³.
Additionally, this technology supports physiologically relevant 3D in vitro models, such as scaffold- or hydrogel-based structures², spheroids⁵, and organoids⁶. By creating 3D models of cells and surrounding tissues, researchers recapitulate the complex mechanical and biochemical properties of tumors in vivo, optimize therapeutic delivery methods, and discover potential cancer therapies⁷. This application note describes the mechanical properties of a bladder tumor and surrounding areas using Pavone technology.
Mechanical characterization of bladder tumor
Pavone is a powerful tool for characterizing the mechanical fingerprints of bladder tumors. The deposition of ECM components, mainly collagen, reduces tissue compliance in bladder tumors. Therefore, mechanical testing and characterization of ECM properties in bladder tumors provide valuable insights into the development and progression of the disease and potential therapeutic targets⁴,⁸.
Here, we mechanically evaluated an orthotopic rat bladder tumor model using Pavone. This study was conducted in collaboration with Massimo Alfano’s research group at Ospedale San Raffaele. The AY-27 rat bladder transitional cell carcinoma cell line was instilled intravesically into a rat to induce a bladder tumor. After four weeks, the bladder tissue was prepared for histology and mechanical testing.
For that, the bladder was instilled with a cryoprotectant (OCT compound) through a catheter before explanting and snap-freezing them. Then, pairs of adjacent 10 and 50 μm thick tissue sections from bladder regions were prepared using a microtome cryostat. The 10 μm thick frozen sections were thawed, formalin-fixed, and hematoxylin–eosin (H&E) stained for histological analyses. For mechanical analysis, 50 μm thick fresh-frozen sections were then thawed in PBS on polarized superfrost glass slides to remove the cryoprotectant prior to mechanical testing.
To preserve the hydrated conditions during measuring, plastic rings were attached to the glass slides by applying a small amount of oil to the outer edge (Figure 1). All procedures and studies involving animals were performed under protocols approved by the IRCCS Ospedale San Raffaele Animal Care and Use Committee and national and international standard guidelines.

Bladder tissue slice placed in the Pavone nanoindenter. To measure in hydrated conditions, plastic rings were attached to the glass slides by applying a small amount of oil to the outer edge.
The mechanical properties of bladder tumors were assessed using a probe with a stiffness of 0.5 N/m and a tip radius of 10 μm. The sample was immersed in saline solution during nanoindentation experiments. According to histological analysis, Young’s modulus was measured at four locations: tumor, below the tumor, transition zone, and unaffected area. Our findings revealed high tissue mechanical heterogeneity, in which stiffness correlated with the distance from the tumor area (Figure 2). The tumor area exhibited higher stiffness than the unaffected area. Interestingly, the transition zone between the tumor and the unaffected area underwent mechanical remodeling. Besides, by analyzing two different sites from a single bladder, we confirmed that the pattern was consistent across locations, as shown in Figures 2 and 3.
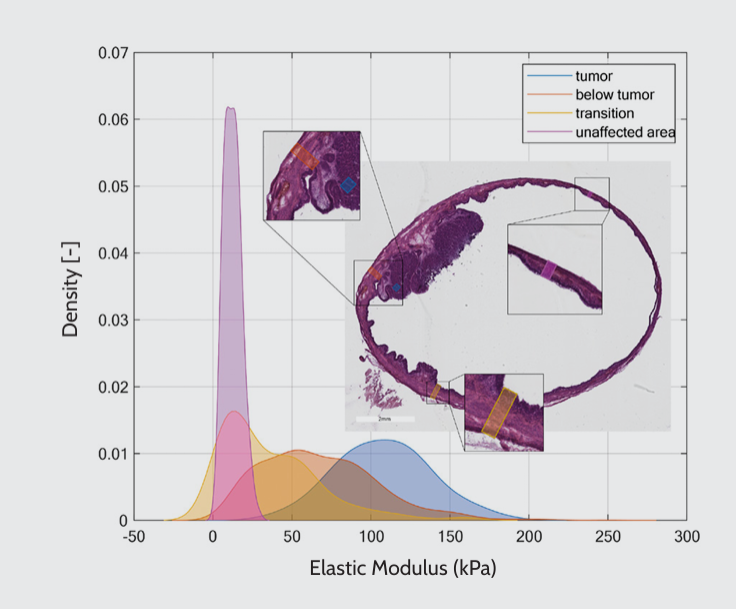
Mechanical characterization of the bladder. The plot presents the kernel density estimation of Young’s modulus for four different bladder regions in an orthotopic rat bladder tumor model: tumor, below the tumor, transition zone, and unaffected area. The corresponding histological figure displays an H&E-stained bladder tissue section, highlighting the locations of the four regions mentioned above.
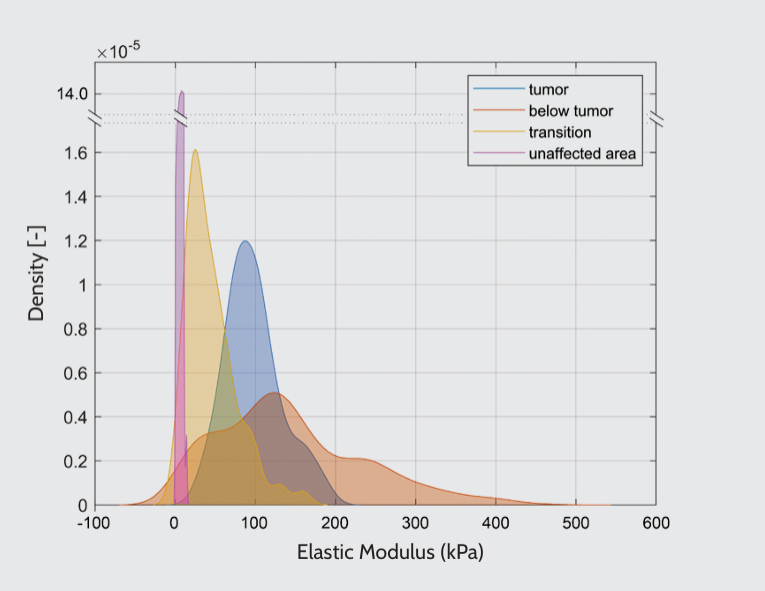
Kernel density estimation of Young’s modulus for two different sites within a single bladder. The data were obtained from two regions within a tissue slice, with a distance of 300 μm separating them. The distributions of mechanical properties differed, but the stiffness trend was consistent as a function of the location.
Conclusion
Our accurate and non-destructive mechanical assessment method identified that the tumor and the peri-tumoral region have distinctive mechanical features compared to healthy tissue. Tumors progressively compromise the mechanical behavior of tissues, making mechanics a potential label-free biomarker for disease development. Understanding the association between mechanical features and clinical manifestations in bladder cancer will contribute to prognostic classification and novel therapies. In light of that, the Pavone is a promising technology for high-throughput cancer research.
References
1 Cox T. R, Erler J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011; 4(2):165–78. doi: 10.1242/dmm.004077.
2 van Tienderen G. S, Conboy J, Muntz I, Willemse J, Tieleman J, Monfils K, Schurink I. J, Demmers J. A. A, Doukas M, Koenderink G. H, van der Laan L. J. W, Verstegen M. M. A. Tumor decellularization reveals proteomic and mechanical characteristics of the extracellular matrix of primary liver cancer. Biomater Adv. 2023 Mar;146:213289. doi: 10.1016/j.bioadv.2023.213289.
3 Narciso M, Martinez A, Junior C, Diaz-Valdivia N, Ulldemolins A, Berardi M, Neal K, Navajas D, Farre R, Alcaraz J, Almendros I, Gavara N. Lung micrometastases display ECM depletion and softening while macrometastases are 30-fold stiffer and enriched in fibronectin. Cancers (Basel). 2023 Apr 21;15(8):2404. doi: 10.3390/cancers15082404.
4 Martinez-Vidal L, Chighizola M, Berardi M, Alchera E, Locatelli I, Pederzoli F, Venegoni C, Luciano R, Milani P, Bielawski K, Salonia A, Podesta A, Alfano M. Micro-mechanical fingerprints of the rat bladder change in actinic cystitis and tumor presence. Commun Biol. 2023 Feb 24;6(1):217. doi: 10.1038/s42003-023-04572-0.
5 Zhang Y, Peng L, Hu K, Gu N. Stress relaxation-induced colon tumor multicellular spheroid culture based on biomimetic hydrogel for nanoenzyme ferroptosis sensitization evaluation. Adv Healthc Mater. 2023 Jan;12(3):e2202009. doi: 10.1002/adhm.202202009.
6 van Tienderen G. S, Rosmark O, Lieshout R, Willemse J, de Weijer F, Elowsson Rendin L, Westergren-Thorsson G, Doukas M, Groot Koerkamp B, van Royen M. E, van der Laan L. J, Verstegen M. M. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023 Mar 1;158:115–131. doi: 10.1016/j.actbio.2022.11.038.
7 Karimnia V, Stanley M. E, Fitzgerald C. T, Rizvi I, Slack F. J, Celli J. P. Photodynamic stromal depletion enhances therapeutic nanoparticle delivery in 3D pancreatic ductal adenocarcinoma tumor models. Photochem Photobiol. 2023 Jan;99(1):120–131. doi: 10.1111/php.13663.
8 Huang X. Z, Zhou A. Y, Liu M. W, Zhang Y, Xu P. Shear wave elasticity differentiation between low- and high-grade bladder urothelial carcinoma and correlation with collagen fiber content. J Ultrasound Med. 2021 Jan;40(1):113–122. doi: 10.1002/jum.15381.

Tumors are conditions characterized by the abnormal growth of cells, usually stemming from genetic mutations. This process can lead to severe complications in organs such as the liver, lungs, and heart. Therefore, it becomes paramount to understand and track tumor progression in disease modeling.
Measuring matrix stiffness
In response to this challenge, our nanoindenters offer a unique solution. They empower researchers to measure the mechanical properties of tissues, providing valuable data that can serve as mechanical biomarkers for the disease. This represents a significant advancement in the field, especially considering that traditional methods of assessing tumors often rely on invasive procedures or indirect measures.
We provide application notes that delve into practical cases where researchers have utilized our nanoindentors in tumor research. Importantly, these notes highlight how these tools can accurately measure the stiffness of tumorous tissues and cells. This information is crucial because an increase in tissue stiffness (matrix stiffness) is a hallmark of tumor progression. Furthermore, the applications of our nanoindenters extend beyond cancer research. They find use in a variety of other fields, including materials science and engineering, where understanding the mechanical properties of materials is essential.

