Pavone’s Environmental Control Module for long-term experiments on living samples
By Giulia Pilia and Fabiany da Costa Gonçalves
© 2023Optics11Life B.V.
Living cultures and biomaterials are sensitive to their surroundings, where fluctuations in temperature, humidity, and gas composition can impact their viability and integrity. Therefore, maintaining precise control over these parameters during experimental procedures, including mechanical characterization, is essential to ensure the reliability and relevance of the outcomes. To address this concern, Pavone combines automated mechanical characterization with the control of various environmental parameters, such as temperature, humidity, and CO₂, to reproduce near-physiological conditions during biological and soft material measurements. This technical note describes how Pavone, combined with the Environmental Control Module, maintains a well-controlled environment for long-term experiments on living samples.
Introduction
Cultivating cells outside the body requires a controlled environment, typically within a cell culture incubator. For most mammalian cells, the main settings are a temperature of 37°C, relative humidity of 95% to minimize evaporation and condensation, and 5% CO₂ to maintain a stable physiological pH¹–³. Some biomaterials that mimic the extracellular microenvironment of native tissue, such as hydrogels, can also respond to these environmental factors⁴.
Considering these requirements, maintaining cellular viability or biomaterial stability for long-term experiments outside the incubator can be challenging. To avoid significant divergence from physiological parameters, methods for mechanical characterization of biological and soft materials must provide optimal environmental conditions. To address this concern, Pavone combines automated mechanical characterization with environmental control, allowing non-destructive characterization of soft materials for large and extended experiments.
Pavone’s Environmental Control Module reproduces near-physiological conditions during biological and soft material measurements. The temperature module ensures a consistent and stable temperature at physiological levels. An adjustable system enables precise regulation of conditions between 35°C and 50°C. The CO₂ and humidity modules provide an incubator-like environment, recreating the optimal settings to maintain cell and tissue viability in extended experiments. CO₂ levels can be set between 0% and 5%, and relative humidity can be increased up to 95%. Finally, the user-friendly software interface provides effortless access and control of all components.
As a proof of concept, the experiments presented in this technical note assess the cellular response to controlled conditions in Pavone’s Environmental Control Module and in a standard incubator. This study was conducted in collaboration with the Department of Biomedical Engineering, Department of Biochemistry, Cardiovascular Research Institute Maastricht (CARIM), and Heart and Vascular Center, Maastricht University Medical Center.
Materials and methods
A. Cell culture
Vascular smooth muscle cells (VSMCs) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) GlutaMAX supplemented with 1% penicillin/streptomycin (P/S), while primary aortic adventitial fibroblasts (AoAFs) were cultured in M199 supplemented with 1% P/S and 1% Fungizone. Cells were seeded in parallel in 48-well plates and maintained in a standard incubator or a Pavone system equipped with an Environmental Control Module, set to 37°C with 5% CO₂ and 95% humidity. Proliferative and apoptotic cells were assessed following overnight incubation (16 h).
B. EdU cell proliferation assay
5-Ethynyl-2′-deoxyuridine (EdU) cell proliferation assay was conducted to detect the proliferation of VSMCs (accelerated proliferative rate) and AoAFs (slow proliferative rate). Cells were exposed to 20 µM EdU solution, incubated, and then fixed in 4% formaldehyde. After fixation, EdU incorporation was detected by fluorescent labeling via Click-iT chemistry with an Alexa Fluor™ 488 dye, and cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI).
Labeled EdU and DAPI double staining of each cell culture was evaluated by an EVOS Invitrogen imaging system with filter sets for GFP and DAPI. EdU-positive and DAPI-positive cells per field (magnification, 10×) were counted in 3 randomly selected areas per well (n = 2–4 wells). The percentage of proliferative cells was calculated by comparing EdU-stained cells to all DAPI-stained cells.
C. Cell apoptosis detection
Annexin V–FITC/Hoechst staining was employed to detect VSMC and AoAF apoptosis. Cells were incubated with 1 µg/ml Annexin V–FITC in binding buffer and counterstained with Hoechst 33342 after staining. Fluorescent Annexin and Hoechst double staining of each cell culture was evaluated by an EVOS Invitrogen imaging system with filter sets for FITC and DAPI. Annexin V-positive and Hoechst-positive cells per field (magnification, 10×) were counted in 3 randomly selected areas per well (n = 2–4 wells). The percentage of apoptotic cells was calculated by comparing Annexin V–stained cells to all Hoechst-stained cells.
D. pH measurement
The pH of the culture medium (M199 plus supplements) was determined using a calibrated pH meter before and after incubation. These measurements were performed on a medium without cells to avoid the confounding influence of cell metabolic activity. The variation in pH values (ΔpH) was quantified as the difference between initial and final pH values (n = 6–12 wells).
E. Statistics
Logistic regression analysis was used to determine the impact of environmental control (Pavone system versus standard incubator) on cell proliferation and apoptosis percentages (level of significance, P < 0.05).
Results
We monitored the cellular response to controlled conditions in a standard incubator and in Pavone’s Environmental Control Module. After overnight incubation, we assessed cell proliferation and apoptosis in VSMCs and AoAFs. As an additional control, we measured the variation in pH values of the culture medium in equivalent settings.
First, we examined the colocalization of EdU labeling and DAPI staining in VSMCs, characterized by an accelerated proliferative rate, and AoAFs, characterized by a slow proliferative rate. The percentage of EdU-positive VSMCs was statistically lower when incubated in the Pavone system than in the standard incubator (OR = 0.6373, 95% CI: 0.5376–0.7554, P < 0.0001), but this difference was negligible (Figure 1A). There was no statistical significance in AoAF proliferation between the two systems (P = 0.07) (Figure 1B).
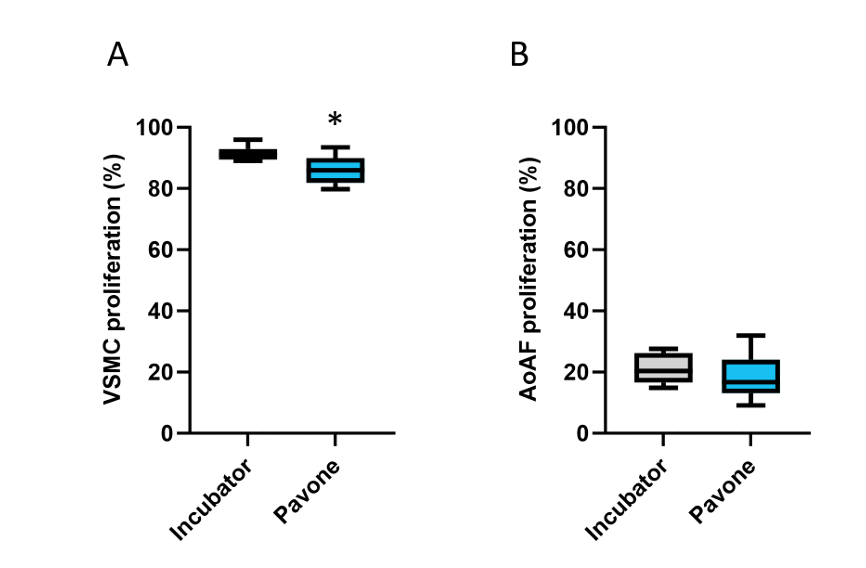
Cell proliferation in the standard incubator and the Pavone’s Environmental Control Module after overnight incubation. (A) Percentage of proliferative VSMC. (B) Percentage of proliferative AoAF. * P < 0,0001.
Then, we evaluated the co-occurrence of Annexin V–FITC and Hoechst in cell cultures. Imaging analysis revealed no statistically significant difference in the percentage of apoptotic cells in the standard incubator and the Pavone system for VSMCs (P = 0.50) and AoAFs (P = 0.94) (Figure 2).

Apoptotic cells in the standard incubator and the Pavone’s Environmental Control Module after overnight incubation. (A) Percentage of apoptotic VSMC. (B) Percentage of apoptotic AoAF.
Finally, we determined the ΔpH of the culture medium before and after incubation. The pH of the culture medium increased from 7.40 ± 0.04 to 7.47 ± 0.04 with ΔpH = 0.07 in the standard incubator and from 7.44 ± 0.04 to 7.63 ± 0.09 with ΔpH = 0.19 in the Pavone system (Figure 3).
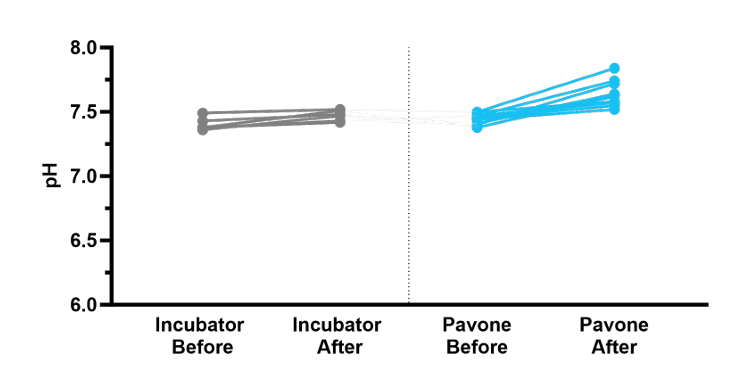
The pH of the culture medium before and after incubation.
Conclusion
- Cell cultures in the Pavone system exhibited proliferation and apoptotic percentages comparable to those in the standard incubator.
- The culture medium in the Pavone system displayed a pH alteration comparable to that in the standard incubator.
Uncontrolled changes in the culturing environment can impact cell viability and act as confounding factors in the analysis. The experimental conditions must be highly reproducible and comparable to standard culturing practices. Thus, Pavone’s Environmental Control Module allows optimal cellular conditions for the mechanical characterization of living cells in extended experiments. The temperature, CO₂, and humidity controls reproduce incubator-like conditions during biological measurements.
Acknowledgements
We thank the research groups of Dr. Koen Reesink and Prof. Leon Schurgers, and particularly Pepijn Saraber, for their assistance in conducting the experiments at Maastricht University Medical Center. Part of the work is financially supported by Public Private Partnership Allowance made available by Health-Holland, Top-Sector Life Sciences and Health, to the Association of Collaborating Health Foundations (SGF) to stimulate public–private partnerships, and by ZonMW, grant LSHM21078-SGF “CELLSYSTEMICS.”
References
- Li L, et al. Progress on preparation of pH/temperature-sensitive intelligent hydrogels and applications in target transport and controlled release of drugs. International Journal of Polymer Science. 2021 Jan;1340538.
- Triaud F, et al. Evaluation of automated cell culture incubators. SLAS Technology. 2003 Dec;8(6):82–86.
- Tayebi-Khorami M, et al. Construction of a CO₂ incubator for cell culture with capability of transmitting microwave radiation. J Med Signals Sens. 2022 May 12;12(2):127–132.
- Lo C. M, et al. pH changes in pulsed CO₂ incubators cause periodic changes in cell morphology. Exp Cell Res. 1994 Aug;213(2):391–7.

