by Optics11 Life
© 2024 Optics11 Life B.V.
The use of 3D-engineered muscles as an in vitro model for disease modeling and drug screening has increased, as they provide essential knowledge of the tissues’ contractile force and electrophysiological properties. Thus, there is a need for a standardized, unbiased, robust, and automatic method to analyze their physiological features. In this application note, we introduce Cuore, an automated force measurement platform for 3D tissue-engineered skeletal and cardiac muscle.
Introduction
Tissue-engineered 3D skeletal and cardiac constructs are increasingly becoming state-of-the-art for in vitro models, supplementing traditional 2D cultures and animal models¹. These 3D models provide a more physiologically relevant environment for cell growth, maturation, and differentiation, where direct contractile force and electrophysiological properties integrate the heterogeneous functions of multiple cell types². The assessment of contractility, whether spontaneous or induced by electrical stimulation, is crucial for investigating muscle physiology and disease progression or treatment. However, current systems face limitations in throughput and sensitivity to absolute force measurement³. With the increased use of engineered tissue models, an unbiased, robust, and automated system is fundamental to analyzing muscle contractile properties in a physiological environment.
Here, we present a continuous and automated force measurement of 3D tissue-engineered skeletal muscles (3D-TESMs) using Cuore, a 3D contraction force platform of Optics11 Life. This study was conducted in collaboration with the Departments of Clinical Genetics and Pediatrics, Molecular, and Stem Cell Laboratory at Erasmus Medical Center and published in Advanced Materials Technologies³.
Measuring contraction of 3D-TESMs
The contractile force of 3D-TESMs generated from three independent induced pluripotent stem cell (hiPSC) lines with different myogenic potentials was measured with Cuore. The forces were assessed continuously in a 24-well plate within the cell incubator from D7 to D14 post-differentiation.
The optimal combination to promote twitch and tetanic contractions in 3D-TESMs to characterize tissue contractile properties was identified using integrated electrical pulse stimulation (EPS) by Cuore. Twitch and tetanic force quantification revealed variations in peak force and time-to-peak force among the three cell lines (Figure 1A–C). Similarly, asynchronous contractions (spontaneous twitch-like contractions followed by a few EPS-induced twitches) were line- and time-dependent features of 3D-TESMs (Figure 1D–F). These findings demonstrated variability in muscle response based on different hiPSC lines over time, serving as a developmental signature of cell lines derived from different donors.
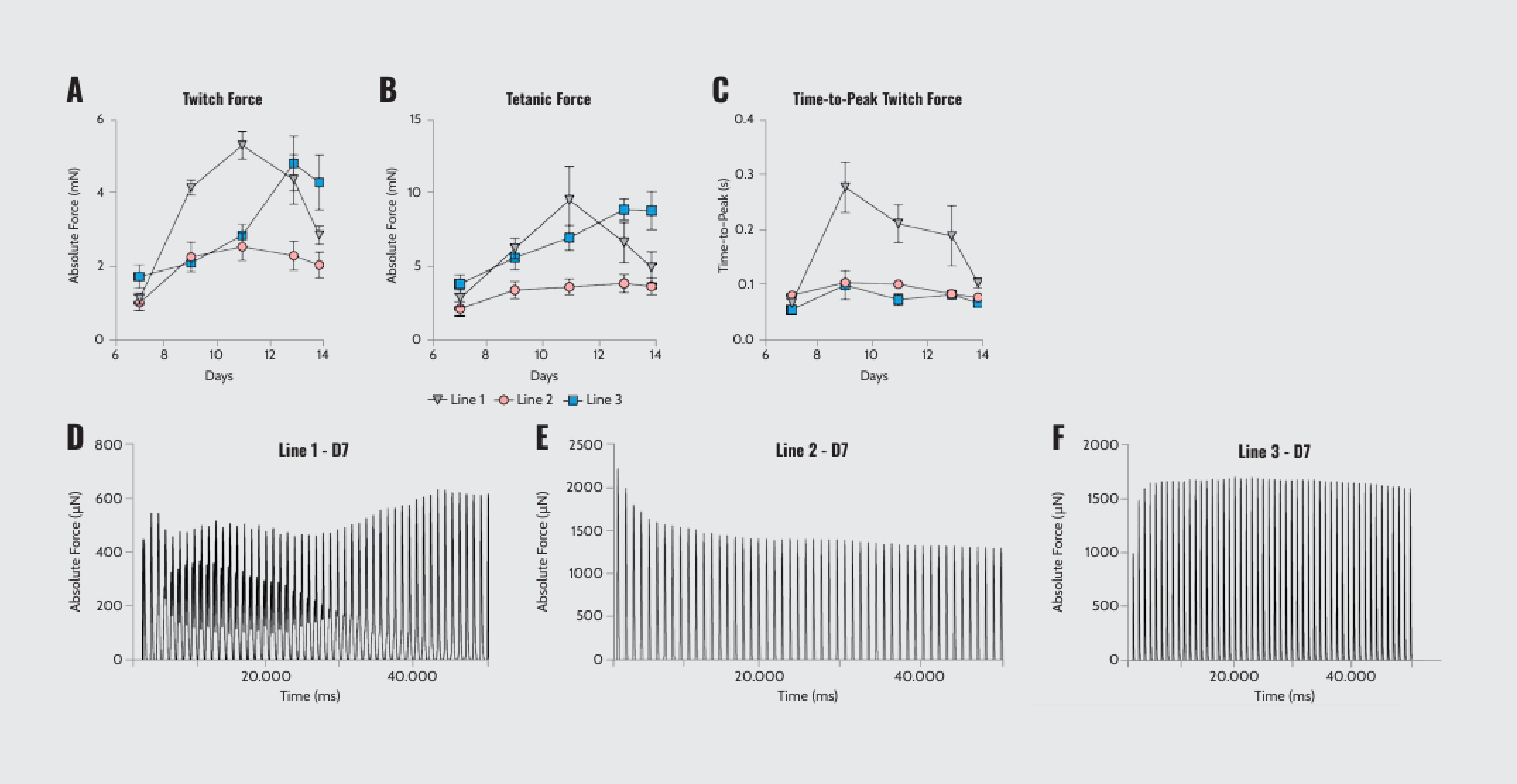
Peak force, time-to-peak force, and asynchronous contraction of 3D-TESMs. (A, B) Absolute twitch and tetanic force (mN) produced by the three lines of MPs-derived 3D-TESMs from D7 to D14 post-differentiation. (C) Quantification of time-to-peak twitch force (ms) for the three lines. (D–F) Representative force curves showing detection of asynchronous patterns of contractility at D7 post-differentiation.
Note: Adapted from “Real-time and Multichannel Measurement of Contractility of hiPSC-Derived 3D Skeletal Muscle using Fiber Optics-Based Sensing”, Iuliano, A., 2023, Adv. Mater. Technol., 8, 2300845. doi: 10.1002/admt.202300845. © 2023 The Authors. Advanced Materials Technologies published by Wiley-VCH GmbH.
To test the effects of drugs on the 3D-TESMs, short- and long-term contractions were measured after acute or chronic administration of clenbuterol. 3D-TESMs from different hiPSC lines showed variable acute responses to clenbuterol (Figure 2A–C). Clenbuterol caused a significant reduction in force in all lines, but the extent varied. Notably, all lines recovered entirely after the drug was washed out, with some lines even showing an increase in force. Besides, chronic treatment with clenbuterol caused unique patterns of force reduction in hiPSC lines (Figure 2D–F). Unveiling treatment response variability is essential for the effectiveness of an in vitro model in relevant drug investigations.
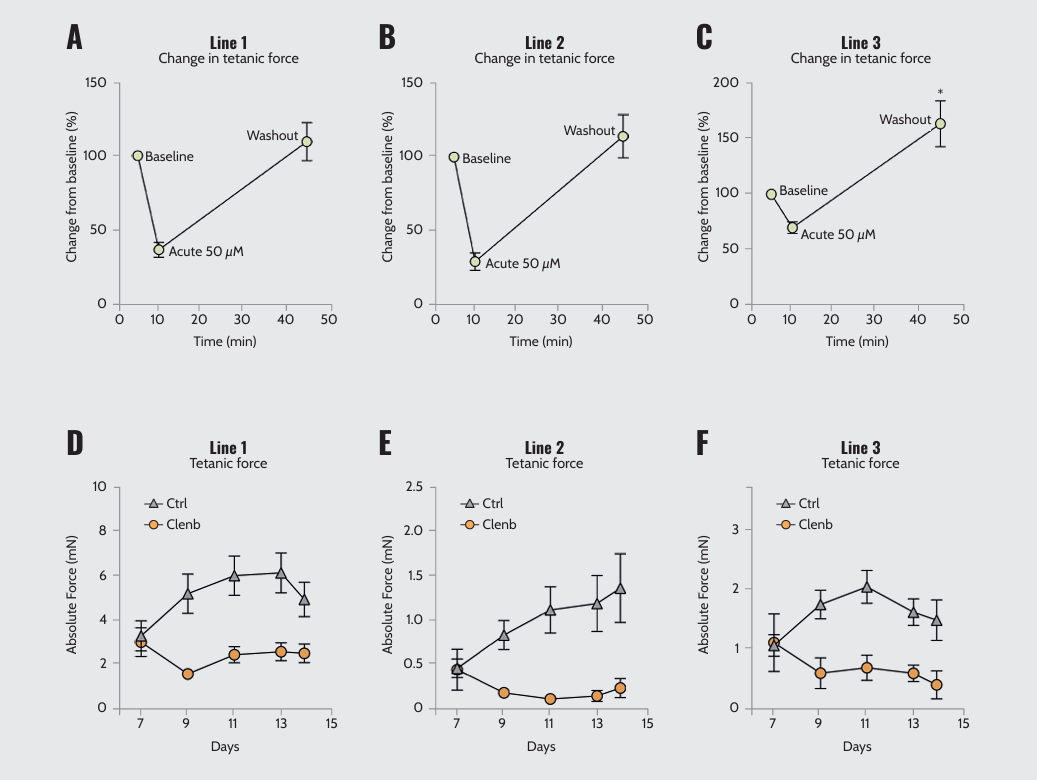
Acute and chronic responses of 3D-TESMs from different lines to clenbuterol. Relative tetanic force change from baseline in the three lines after acute administration of 50 μM clenbuterol. Absolute twitch force of the three lines with (Clenb) or without (Ctrl) chronic administration of 10 μM clenbuterol for one week.
Note: Adapted from “Real-time and Multichannel Measurement of Contractility of hiPSC-Derived 3D Skeletal Muscle using Fiber Optics-Based Sensing”, Iuliano, A., 2023, Adv. Mater. Technol., 8, 2300845. doi: 10.1002/admt.202300845. © 2023 The Authors. Advanced Materials Technologies published by Wiley-VCH GmbH.
Conclusion
Cuore offers precise and reproducible analysis of in vitro 3D contractile tissues. Its automated force recording with integrated electrical stimulation introduces novel opportunities for investigating the functionality and maturation of 3D-TESMs over time and under physiological conditions. The ability to track real-time changes in contractility as a live biomarker makes Cuore an attractive choice for novel therapy development or drug testing with in vitro tissue-engineered skeletal and cardiac muscle models.
Acknowledgements
We thank Dr. Pim Pijnappel and his research team at Erasmus Medical Center, especially Alessandro Iuliano, for their invaluable scientific insights and expertise in developing the experiments described in this application note.
DISCLAIMER
We have obtained permission from the copyright owners to use the adapted figures in this application note.
References
1 Khodabukus A, et al. In vitro tissue-engineered skeletal muscle models for studying muscle physiology and disease. Adv Healthc Mater. 2018 Aug; 7(15):e1701498. doi: 10.1002/adhm.201701498.
2 Weinberger F, et al. Engineering cardiac muscle tissue: A maturing field of research. Circ Res. 2017 Apr 28; 120(9):1487–1500. doi: 10.1161/CIRCRESAHA.117.310738.
3 Iuliano A, et al. Real-time and multichannel measurement of contractility of hiPSC-derived 3D skeletal muscle using fiber optics-based sensing. Adv. Mater. Technol. 2023; 8, 2300845. doi: 10.1002/admt.202300845.

Key takeaways:
- Introduction of Cuore: Optics11 Life’s automated platform for measuring contraction kinetics in 3D-engineered muscle tissues.
- Enhanced Research Capability: Enables precise, automated analysis of muscle physiology for both skeletal and cardiac muscle models.
- Application in Disease and Drug Screening: Ideal for in vitro disease modeling and drug response analysis, providing vital physiological insights.
- Innovative Features: Continuous and real-time measurement capabilities within a cell incubator environment.
- Research and Development: Serves as a critical tool in the development of new therapies and drug testing, demonstrating variability and response in human-induced pluripotent stem cell-derived muscle tissues.

